Tryptophan Synthase Uses an Atypical Mechanism To Achieve Substrate Specificity.
Buller, A.R., van Roye, P., Murciano-Calles, J., Arnold, F.H.(2016) Biochemistry 55: 7043-7046
- PubMed: 27935677
- DOI: https://doi.org/10.1021/acs.biochem.6b01127
- Primary Citation of Related Structures:
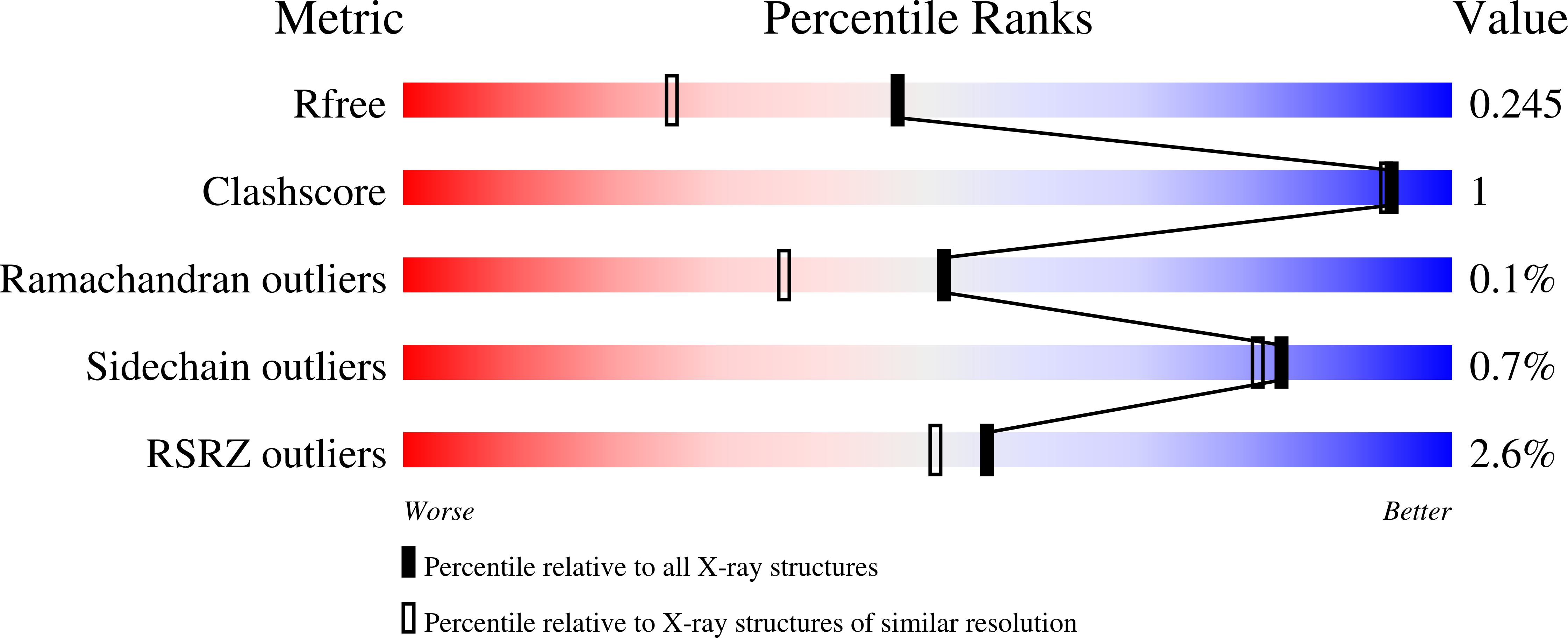
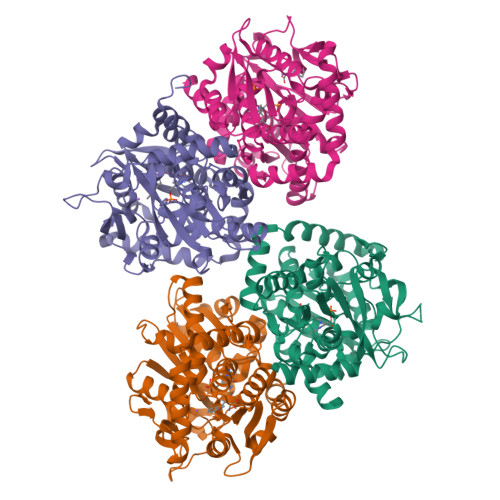
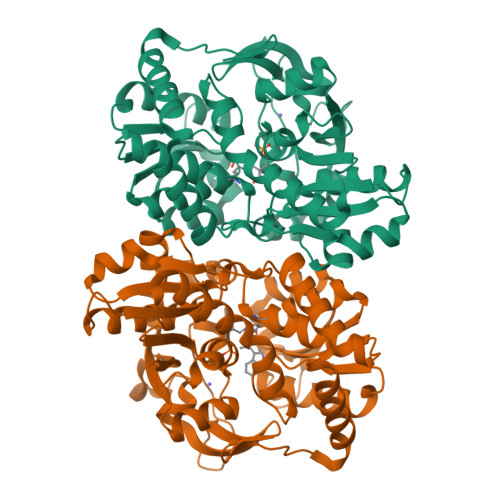
5T6M - PubMed Abstract:
Tryptophan synthase (TrpS) catalyzes the final steps in the biosynthesis of l-tryptophan from l-serine (Ser) and indole-3-glycerol phosphate (IGP). We report that native TrpS can also catalyze a productive reaction with l-threonine (Thr), leading to (2S,3S)-β-methyltryptophan. Surprisingly, β-substitution occurs in vitro with a 3.4-fold higher catalytic efficiency for Ser over Thr using saturating indole, despite a >82000-fold preference for Ser in direct competition using IGP. Structural data identify a novel product binding site, and kinetic experiments clarify the atypical mechanism of specificity: Thr binds efficiently but decreases the affinity for indole and disrupts the allosteric signaling that regulates the catalytic cycle.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering 210-41, California Institute of Technology , 1200 East California Boulevard, Pasadena, California 91125, United States.