Structural Basis of Furan-Amino Acid Recognition by a Polyspecific Aminoacyl-tRNA-Synthetase and its Genetic Encoding in Human Cells.
Schmidt, M.J., Weber, A., Pott, M., Welte, W., Summerer, D.(2014) Chembiochem 15: 1755
- PubMed: 24737732
- DOI: https://doi.org/10.1002/cbic.201402006
- Primary Citation of Related Structures:
4CS2, 4CS3, 4CS4 - PubMed Abstract:
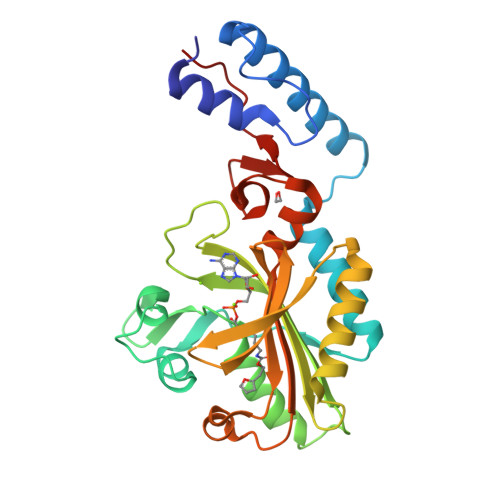
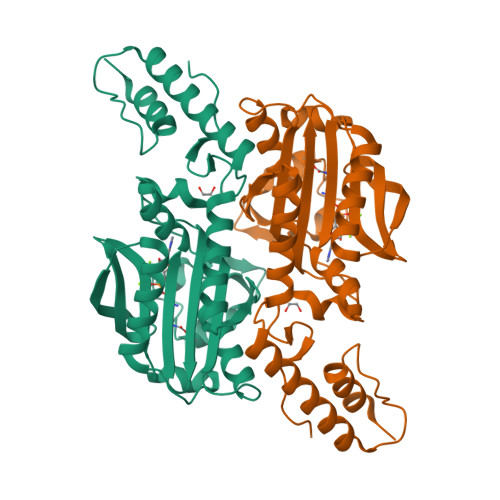
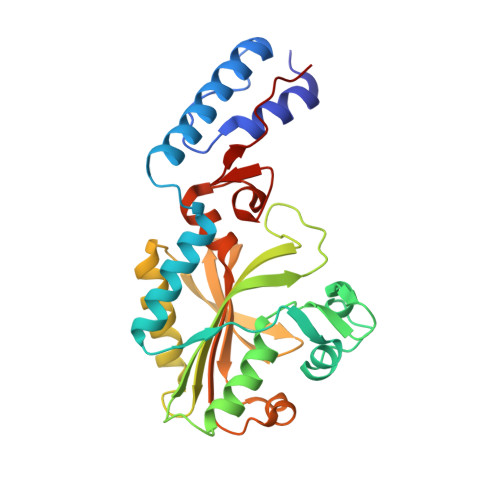
The site-selective introduction of photo-crosslinking groups into proteins enables the discovery and mapping of weak and/or transient protein interactions with high spatiotemporal resolution, both in vitro and in vivo. We report the genetic encoding of a furan-based, photo-crosslinking amino acid in human cells; it can be activated with red light, thus offering high penetration depths in biological samples. This is achieved by activation of the amino acid and charging to its cognate tRNA by a pyrrolysyl-tRNA-synthetase (PylRS) mutant with broad polyspecificity. To gain insights into the recognition of this amino acid and to provide a rationale for its polyspecificity, we solved three crystal structures of the PylRS mutant: in its apo-form, in complex with adenosine 5'-(β,γ-imido)triphosphate (AMP-PNP) and in complex with the AMP ester of the furan amino acid. These structures provide clues for the observed polyspecificity and represent a promising starting point for the engineering of PylRS mutants with further increased substrate scope.
Organizational Affiliation:
Department of Chemistry, University of Konstanz, Universitätsstrasse 10, 78457 Konstanz (Germany).