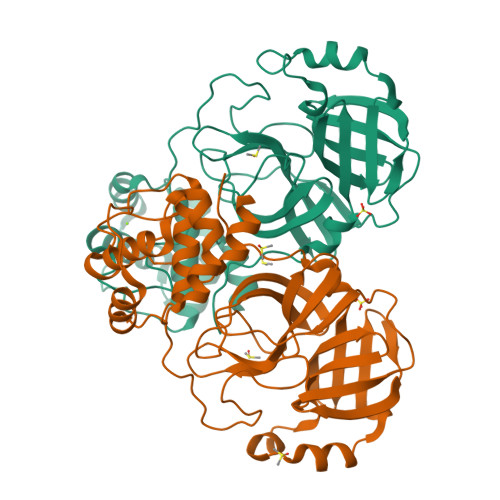
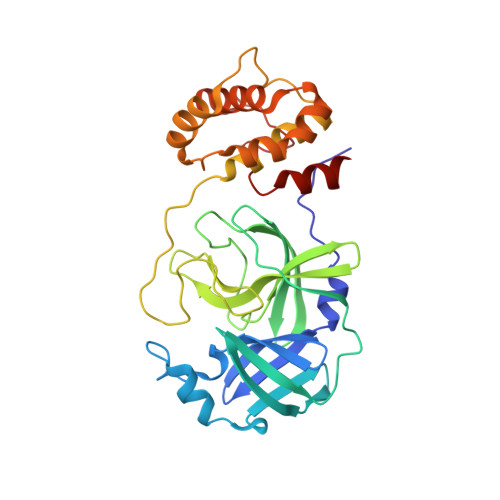
Cryo2RT: a high-throughput method for room-temperature macromolecular crystallography from cryo-cooled crystals.
Huang, C.Y., Aumonier, S., Olieric, V., Wang, M.(2024) Acta Crystallogr D Struct Biol 80: 620-628
- PubMed: 39052318
- DOI: https://doi.org/10.1107/S2059798324006697
- Primary Citation of Related Structures:
7H56, 7H57, 7H58, 7H59, 7H5A, 7H5B, 7H5C, 7H5D, 7H5E, 7H5F, 7H5G, 7H5H, 7H5I, 7H5J, 7H5K, 7H5L, 7H5M, 7H5N, 7H5O, 7H5P, 7H5Q, 7H5R, 7H5S, 7H5T, 7H5U, 7H5V, 7H5W, 7H5X, 7H5Y, 7H5Z, 9FX4, 9FX5, 9FX6, 9FX7 - PubMed Abstract:
Advances in structural biology have relied heavily on synchrotron cryo-crystallography and cryogenic electron microscopy to elucidate biological processes and for drug discovery. However, disparities between cryogenic and room-temperature (RT) crystal structures pose challenges. Here, Cryo2RT, a high-throughput RT data-collection method from frozen crystals that leverages the cryo-crystallography workflow, is introduced. Tested on endothiapepsin crystals with four soaked fragments, thaumatin and SARS-CoV-2 3CL pro , Cryo2RT reveals unique ligand-binding poses, offers a comparable throughput to cryo-crystallography and eases the exploration of structural dynamics at various temperatures.
Organizational Affiliation:
Swiss Light Source, Center for Photon Science, Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland.