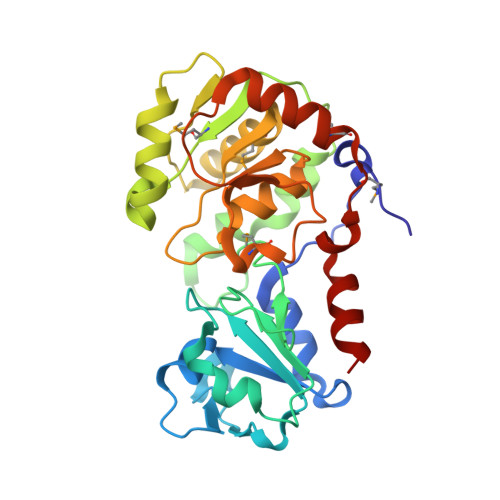
Unveiling the GT114 family: Structural characterization of A075L, a glycosyltransferase from Paramecium bursaria chlorella virus-1 (PBCV-1).
Laugieri, M.E., Speciale, I., Gimeno, A., Lin, S., Byers, B.W., Poveda, A., Nunez-Franco, R., Iturrioz, I., Moure, M.J., Jimenez-Oses, G., Russo-Krauss, I., Notaro, A., Van Etten, J.L., Lowary, T.L., Jimenez-Barbero, J., De Castro, C., Tonetti, M., Rojas, A.L.(2024) Protein Sci 33: e5196-e5196
- PubMed: 39555664
- DOI: https://doi.org/10.1002/pro.5196
- Primary Citation of Related Structures:
8ASA, 8AVQ, 8Q8I - PubMed Abstract:
Protein A075L is a β-xylosyltransferase that participates in producing the core of the N-glycans found in VP54, the major viral capsid protein of Paramecium bursaria chlorella virus-1 (PBCV-1). In this study, we present an X-ray crystallographic analysis of the apo form of A075L, along with its complexes with the sugar donor and with a trisaccharide acceptor. The protein structure shows a typical GT-B folding, with two Rossmann-like fold domains, in which the acceptor substrate binds to the N-terminal region, and the nucleotide-sugar donor binds to the C-terminal region. We propose that the catalytic mechanism follows a direct displacement S N 2-like reaction, where Asp73 serves as a catalytic base that deprotonates the incoming nucleophile of the acceptor, facilitating direct displacement of the UDP with the inversion of the anomeric configuration of the acceptor without metal ion dependence, while the interactions with side chains of Arg158 and Arg208 stabilize the developing negative charge. Using isothermal titration calorimetry, nuclear magnetic resonance spectroscopy, high-performance liquid chromatography, and molecular dynamics simulations, the catalytic activity and specificity of this enzyme have been unraveled.
Organizational Affiliation:
DIMES - Biochemistry Division, University of Genoa, Genoa, Italy.