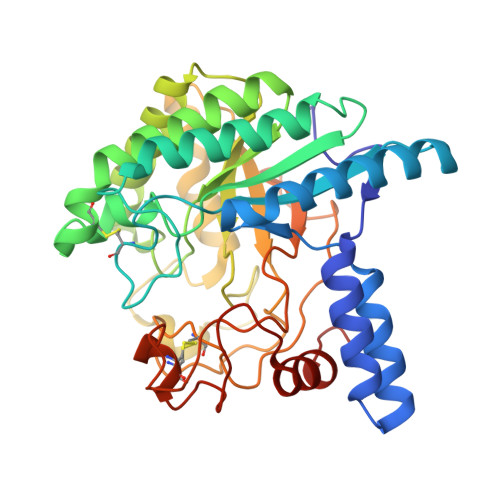
Deprotonated Arginine Controls a Putative Catalytic Base in Invert-ing Family 6 Glycoside Hydrolase
Tachioka, M., Yamaguchi, S., Nakamura, A., Ishida, T., Kusaka, K., Yamada, T., Yano, N., Chatake, T., Tamada, T., Takeda, K., Niwa, S., Tanaka, H., Takahashi, S., Inaka, K., Furubayashi, N., Deguchi, S., Samejima, M., Igarashi, K.To be published.