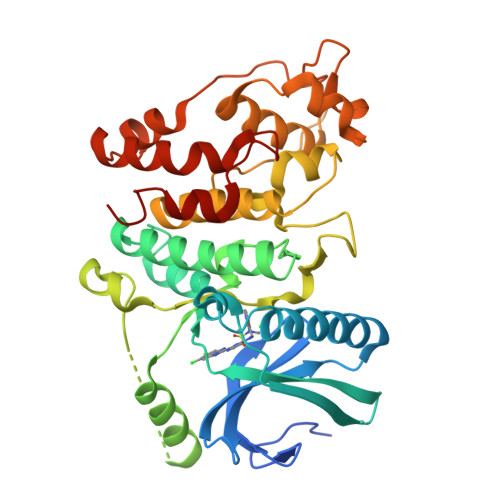
MSC-1186, a Highly Selective Pan-SRPK Inhibitor Based on an Exceptionally Decorated Benzimidazole-Pyrimidine Core.
Schroder, M., Leiendecker, M., Gradler, U., Braun, J., Blum, A., Wanior, M., Berger, B.T., Kramer, A., Muller, S., Esdar, C., Knapp, S., Heinrich, T.(2023) J Med Chem 66: 837-854
- PubMed: 36516476
- DOI: https://doi.org/10.1021/acs.jmedchem.2c01705
- Primary Citation of Related Structures:
7ZKS, 7ZKX - PubMed Abstract:
The highly conserved catalytic sites in protein kinases make it difficult to identify ATP competitive inhibitors with kinome-wide selectivity. Serendipitously, during a dedicated fragment campaign for the focal adhesion kinase (FAK), a scaffold that had lost its initial FAK affinity showed remarkable potency and selectivity for serine-arginine-protein kinases 1-3 (SRPK1-3). Non-conserved interactions with the uniquely structured hinge region of the SRPK family were the key drivers of the exclusive selectivity of the discovered fragment hit. Structure-guided medicinal chemistry efforts led to the SRPK inhibitor MSC-1186 , which fulfills all hallmarks of a reversible chemical probe, including nanomolar cellular potency and excellent kinome-wide selectivity. The combination of MSC-1186 with CDC2-like kinase (CLK) inhibitors showed additive attenuation of SR-protein phosphorylation compared to the single agents. MSC-1186 and negative control ( MSC-5360 ) are chemical probes available via the Structural Genomics Consortium chemical probe program (https://www.sgc-ffm.uni-frankfurt.de/).
Organizational Affiliation:
SGC Frankfurt, Goethe University Frankfurt, Buchmann Institute for Life Sciences (BMLS), Riedberg Campus, Max-von-Laue-Str. 15, 60438 Frankfurt am Main, Germany.