The Antiadhesive Strategy in Crohn's Disease: Orally Active Mannosides to Decolonize Pathogenic Escherichia coli from the Gut.
Alvarez Dorta, D., Sivignon, A., Chalopin, T., Dumych, T.I., Roos, G., Bilyy, R.O., Deniaud, D., Krammer, E.M., de Ruyck, J., Lensink, M.F., Bouckaert, J., Barnich, N., Gouin, S.G.(2016) Chembiochem 17: 936-952
- PubMed: 26946458
- DOI: https://doi.org/10.1002/cbic.201600018
- Primary Citation of Related Structures:
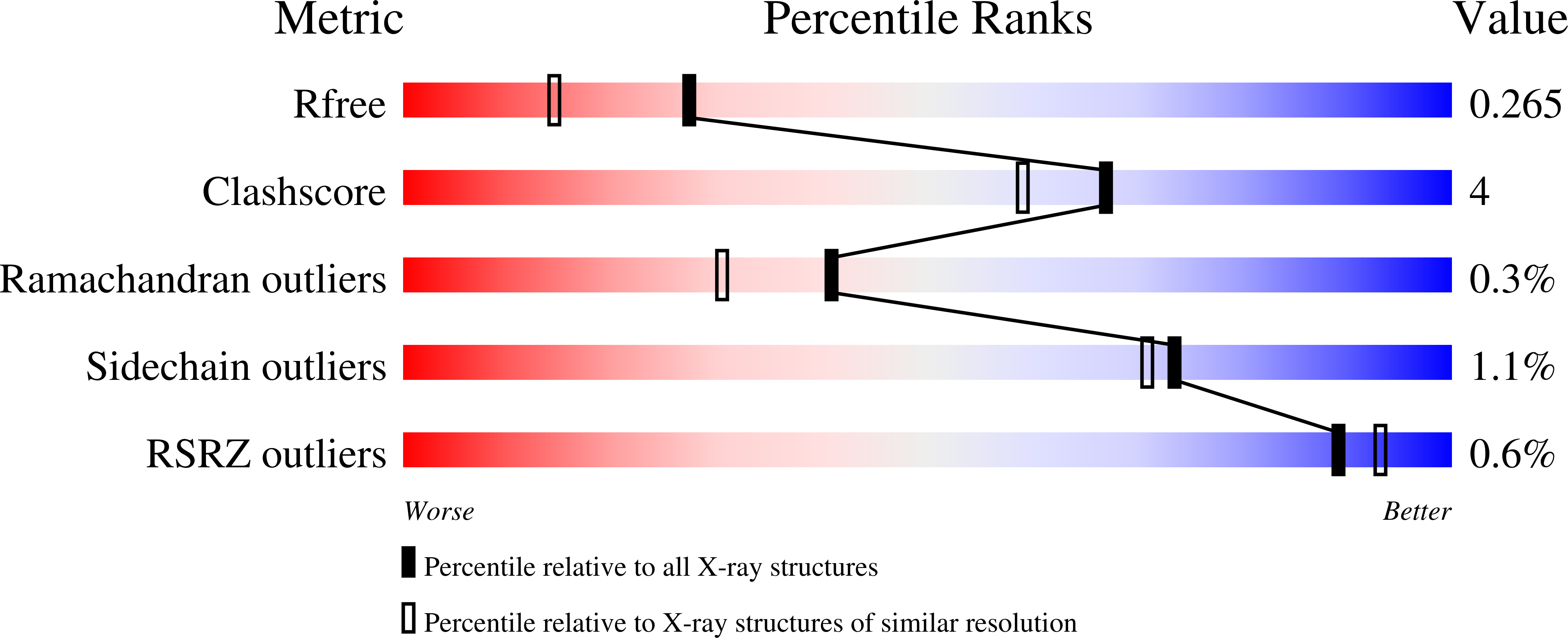
6YHW - PubMed Abstract:
Blocking the adherence of bacteria to cells is an attractive complementary approach to current antibiotic treatments, which are faced with increasing resistance. This strategy has been particularly studied in the context of urinary tract infections (UTIs), in which the adhesion of pathogenic Escherichia coli strains to uroepithelial cells is prevented by blocking the FimH adhesin expressed at the tips of bacteria organelles called fimbriae. Recently, we extended the antiadhesive concept, showing that potent FimH antagonists can block the attachment of adherent-invasive E. coli (AIEC) colonizing the intestinal mucosa of patients with Crohn's disease (CD). In this work, we designed a small library of analogues of heptyl mannoside (HM), a previously identified nanomolar FimH inhibitor, but one that displays poor antiadhesive effects in vivo. The anomeric oxygen atom was replaced by a sulfur or a methylene group to prevent hydrolysis by intestinal glycosidases, and chemical groups were attached at the end of the alkyl tail. Importantly, a lead compound was shown to reduce AIEC levels in the feces and in the colonic and ileal mucosa after oral administration (10 mg kg(-1) ) in a transgenic mouse model of CD. The compound showed a low bioavailability, preferable in this instance, thus suggesting the possibility of setting up an innovative antiadhesive therapy, based on the water-soluble and non-cytotoxic FimH antagonists developed here, for the CD subpopulation in which AIEC plays a key role.
Organizational Affiliation:
LUNAM Université, CEISAM, Chimie et Interdisciplinarité, Synthèse, Analyse, Modélisation, UMR CNRS 6230, 2, rue de la Houssinière, BP 92208, 44322, Nantes Cedex 3, France.