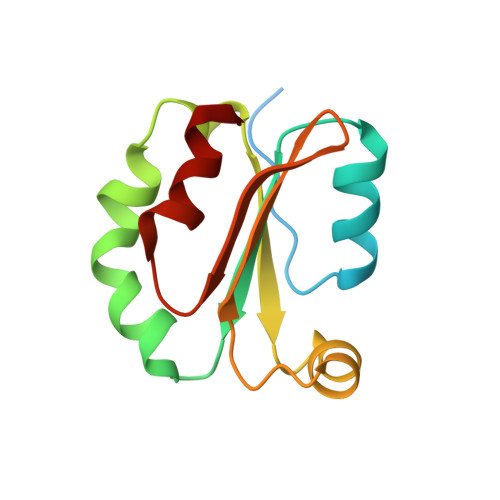
Trxlp, a thioredoxin-like effector from Edwardsiella piscicida inhibits cellular redox signaling and nuclear translocation of NF-kappa B.
Sayed, A., Chakraborty, S., Leung, K.Y., Sugii, S., Mok, Y.K.(2020) Int J Biol Macromol 148: 89-101
- PubMed: 31945434
- DOI: https://doi.org/10.1016/j.ijbiomac.2020.01.114
- Primary Citation of Related Structures:
5ZPV - PubMed Abstract:
Redox signaling and homeostasis are essential for cell survival and the immune response. Peroxiredoxin (Prx) modulates the level of H 2 O 2 as a redox signal through H 2 O 2 decomposition. The redox activity of thioredoxin (Trx) is required as a reducing equivalent to regenerate Prx. Edwardsiella piscicida is an opportunistic Gram-negative enteric pathogen that secretes a novel Trx-like effector protein, ETAE_2186 (Trxlp). Trxlp has unique structural properties compared with other Trx proteins. In enzymatic and binding assays, we confirmed Trxlp to be redox-inactive due to the low reactivity and flexibility of the resolving cysteine residue, C35, at the active site motif " 31 WCXXC 35 ". We identified key residues near the active site that are critical for reactivity and flexibility of C35 by site-directed mutagenesis analysis. NMR titration experiment demonstrated prolong inhibitory interaction of Trxlp with Prx1 resulting in the repression of Prx1-mediated H 2 O 2 decomposition leading to increased ROS accumulation in infected host cells. Increased ROS in turn prevented nuclear translocation of NF-κB and inhibition of NF-κB target genes, leading to bacterial survival and enhanced replication inside host cells. Targeting Trxlp-mediated virulence promises to attenuate E. piscicida infection.
Organizational Affiliation:
Department of Biological Sciences, 16 Science Drive 4, National University of Singapore, 117558, Singapore.