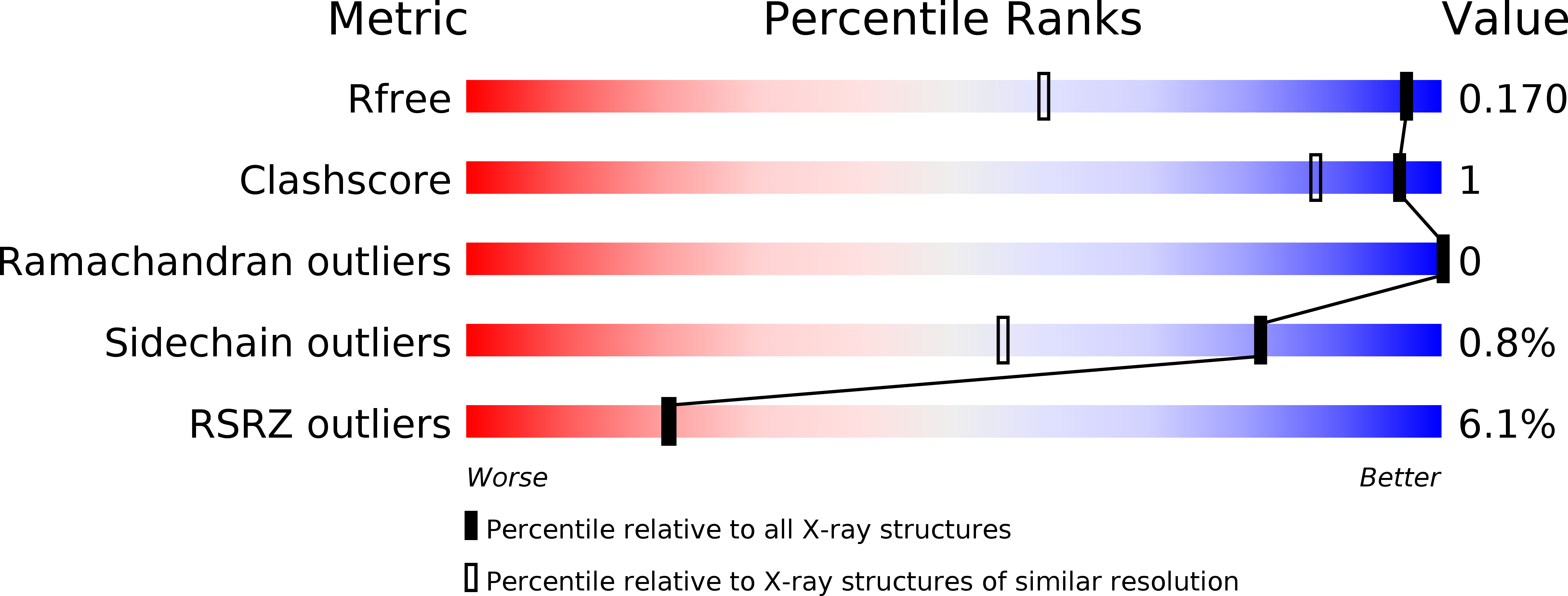
Discovery and characterization of [(cyclopentyl)ethyl]benzoic acid inhibitors of microsomal prostaglandin E synthase-1.
Partridge, K.M., Antonysamy, S., Bhattachar, S.N., Chandrasekhar, S., Fisher, M.J., Fretland, A., Gooding, K., Harvey, A., Hughes, N.E., Kuklish, S.L., Luz, J.G., Manninen, P.R., McGee, J.E., Mudra, D.R., Navarro, A., Norman, B.H., Quimby, S.J., Schiffler, M.A., Sloan, A.V., Warshawsky, A.M., Weller, J.M., York, J.S., Yu, X.P.(2017) Bioorg Med Chem Lett 27: 1478-1483
- PubMed: 28190634
- DOI: https://doi.org/10.1016/j.bmcl.2016.11.011
- Primary Citation of Related Structures:
5T36, 5T37, 5TL9 - PubMed Abstract:
We describe a novel class of acidic mPGES-1 inhibitors with nanomolar enzymatic and human whole blood (HWB) potency. Rational design in conjunction with structure-based design led initially to the identification of anthranilic acid 5, an mPGES-1 inhibitor with micromolar HWB potency. Structural modifications of 5 improved HWB potency by over 1000×, reduced CYP2C9 single point inhibition, and improved rat clearance, which led to the selection of [(cyclopentyl)ethyl]benzoic acid compound 16 for clinical studies. Compound 16 showed an IC 80 of 24nM for inhibition of PGE 2 formation in vitro in LPS-stimulated HWB. A single oral dose resulted in plasma concentrations of 16 that exceeded its HWB IC 80 in both rat (5mg/kg) and dog (3mg/kg) for over twelve hours.
Organizational Affiliation:
Lilly Research Laboratories, A Division of Eli Lilly and Company, Indianapolis, IN 46285, USA. Electronic address: [email protected].