SAR Studies of 5-Aminopyrazole-4-carboxamide Analogues as Potent and Selective Inhibitors of Toxoplasma gondii CDPK1.
Huang, W., Ojo, K.K., Zhang, Z., Rivas, K., Vidadala, R.S., Scheele, S., DeRocher, A.E., Choi, R., Hulverson, M.A., Barrett, L.K., Bruzual, I., Siddaramaiah, L.K., Kerchner, K.M., Kurnick, M.D., Freiberg, G.M., Kempf, D., Hol, W.G., Merritt, E.A., Neckermann, G., de Hostos, E.L., Isoherranen, N., Maly, D.J., Parsons, M., Doggett, J.S., Van Voorhis, W.C., Fan, E.(2015) ACS Med Chem Lett 6: 1184-1189
- PubMed: 26693272
- DOI: https://doi.org/10.1021/acsmedchemlett.5b00319
- Primary Citation of Related Structures:
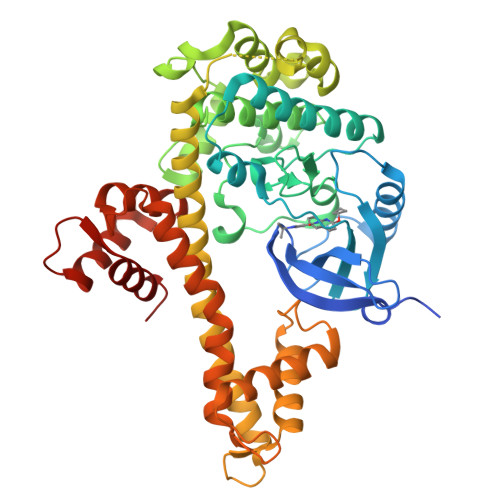
4YJN - PubMed Abstract:
We previously discovered compounds based on a 5-aminopyrazole-4-carboxamide scaffold to be potent and selective inhibitors of CDPK1 from T. gondii . The current work, through structure-activity relationship studies, led to the discovery of compounds ( 34 and 35 ) with improved characteristics over the starting inhibitor 1 in terms of solubility, plasma exposure after oral administration in mice, or efficacy on parasite growth inhibition. Compounds 34 and 35 were further demonstrated to be more effective than 1 in a mouse infection model and markedly reduced the amount of T. gondii in the brain, spleen, and peritoneal fluid, and 35 given at 20 mg/kg eliminated T. gondii from the peritoneal fluid.
Organizational Affiliation:
Department of Biochemistry, University of Washington , Seattle, Washington 98195, United States.