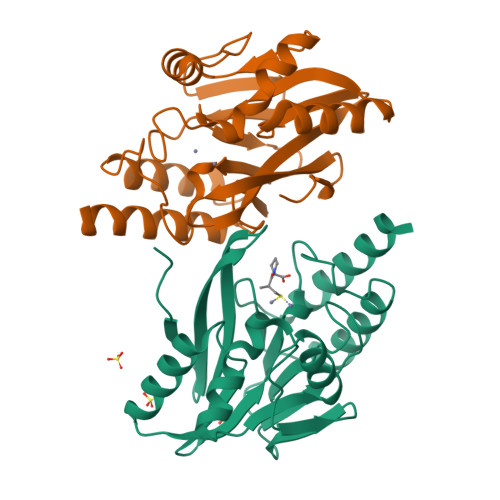
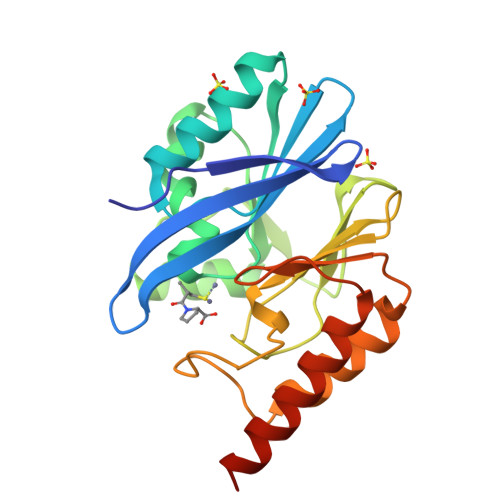
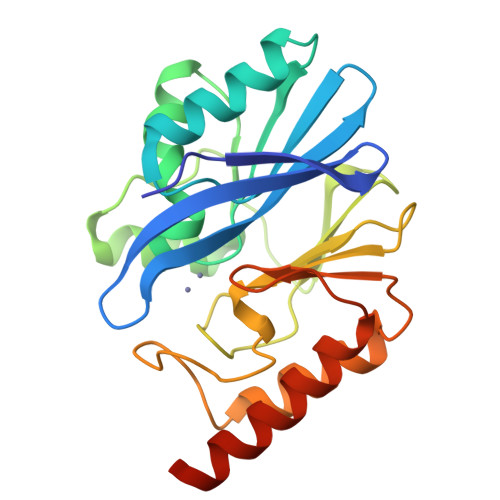
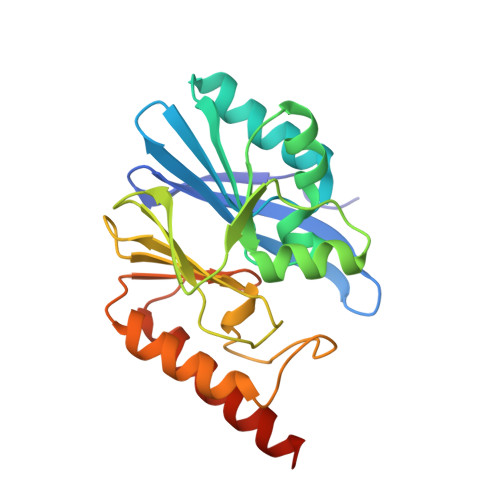
Structural Basis of Metallo-beta-Lactamase Inhibition by Captopril Stereoisomers.
Brem, J., van Berkel, S.S., Zollman, D., Lee, S.Y., Gileadi, O., McHugh, P.J., Walsh, T.R., McDonough, M.A., Schofield, C.J.(2015) Antimicrob Agents Chemother 60: 142-150
- PubMed: 26482303
- DOI: https://doi.org/10.1128/AAC.01335-15
- Primary Citation of Related Structures:
4BZ3, 4C09, 4C1C, 4C1D, 4C1E, 4C1F, 4C1G, 4C1H - PubMed Abstract:
β-Lactams are the most successful antibacterials, but their effectiveness is threatened by resistance, most importantly by production of serine- and metallo-β-lactamases (MBLs). MBLs are of increasing concern because they catalyze the hydrolysis of almost all β-lactam antibiotics, including recent-generation carbapenems. Clinically useful serine-β-lactamase inhibitors have been developed, but such inhibitors are not available for MBLs. l-Captopril, which is used to treat hypertension via angiotensin-converting enzyme inhibition, has been reported to inhibit MBLs by chelating the active site zinc ions via its thiol(ate). We report systematic studies on B1 MBL inhibition by all four captopril stereoisomers. High-resolution crystal structures of three MBLs (IMP-1, BcII, and VIM-2) in complex with either the l- or d-captopril stereoisomer reveal correlations between the binding mode and inhibition potency. The results will be useful in the design of MBL inhibitors with the breadth of selectivity required for clinical application against carbapenem-resistant Enterobacteriaceae and other organisms causing MBL-mediated resistant infections.
Organizational Affiliation:
Department of Chemistry, University of Oxford, Oxford, United Kingdom.