Discovery of a Small-Molecule Type II Inhibitor of Wild-Type and Gatekeeper Mutants of Bcr-Abl, Pdgfralpha, Kit, and Src Kinases: Novel Type II Inhibitor of Gatekeeper Mutants.
Weisberg, E., Choi, H.G., Ray, A., Barrett, R., Zhang, J., Sim, T., Zhou, W., Seeliger, M., Cameron, M., Azam, M., Fletcher, J.A., Debiec-Rychter, M., Mayeda, M., Moreno, D., Kung, A.L., Janne, P.A., Khosravi-Far, R., Melo, J.V., Manley, P.W., Adamia, S., Wu, C., Gray, N., Griffin, J.D.(2010) Blood 115: 4206
- PubMed: 20299508
- DOI: https://doi.org/10.1182/blood-2009-11-251751
- Primary Citation of Related Structures:
4AGW - PubMed Abstract:
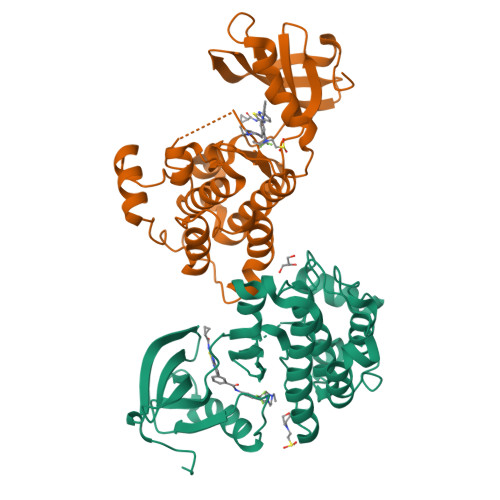
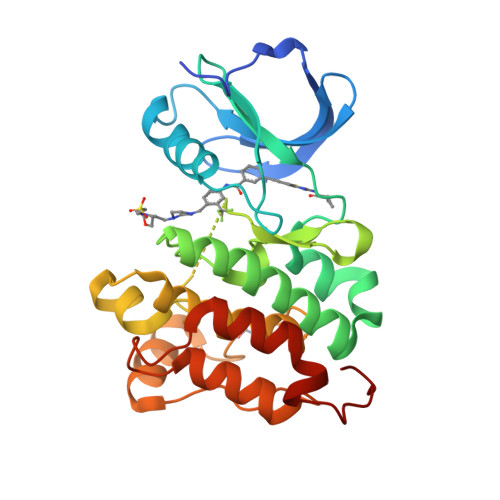
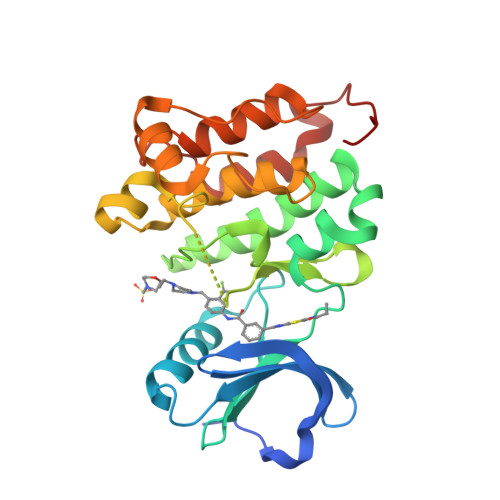
Many clinically validated kinases, such as BCR-ABL, c-Kit, PDGFR, and EGFR, become resistant to adenosine triphosphate-competitive inhibitors through mutation of the so-called gatekeeper amino acid from a threonine to a large hydrophobic amino acid, such as an isoleucine or methionine. We have developed a new class of adenosine triphosphate competitive inhibitors, exemplified by HG-7-85-01, which is capable of inhibiting T315I- BCR-ABL (clinically observed in chronic myeloid leukemia), T670I-c-Kit (clinically observed in gastrointestinal stromal tumors), and T674I/M-PDGFRalpha (clinically observed in hypereosinophilic syndrome). HG-7-85-01 is unique among all currently reported kinase inhibitors in having the ability to accommodate either a gatekeeper threonine, present in the wild-type forms of these kinases, or a large hydrophobic amino acid without becoming a promiscuous kinase inhibitor. The distinctive ability of HG-7-85-01 to simultaneously inhibit both wild-type and mutant forms of several kinases of clinical relevance is an important step in the development of the next generation of tyrosine kinase inhibitors.
Organizational Affiliation:
Department of Medical Oncology/Hematologic Neoplasia, Dana-Farber Cancer Institute, Boston, MA. Ellen_Weisberg@dfci.harvard.edu