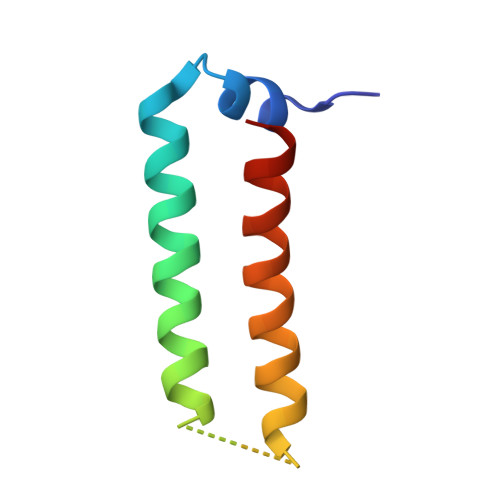
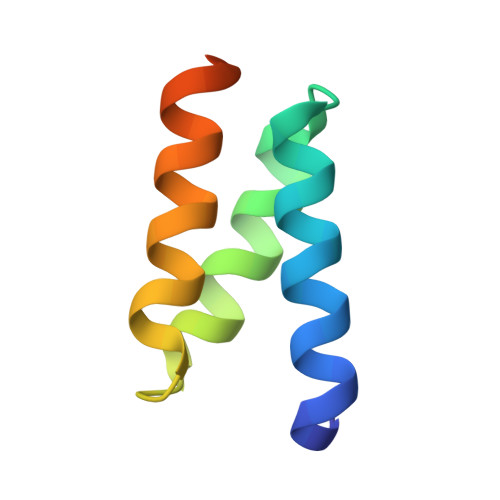
Crystal structure of the heteromolecular chaperone, AscE-AscG, from the type III secretion system in Aeromonas hydrophila
Chatterjee, C., Kumar, S., Chakraborty, S., Tan, Y.W., Leung, K.Y., Sivaraman, J., Mok, Y.K.(2011) PLoS One 6: e19208-e19208
- PubMed: 21559439
- DOI: https://doi.org/10.1371/journal.pone.0019208
- Primary Citation of Related Structures:
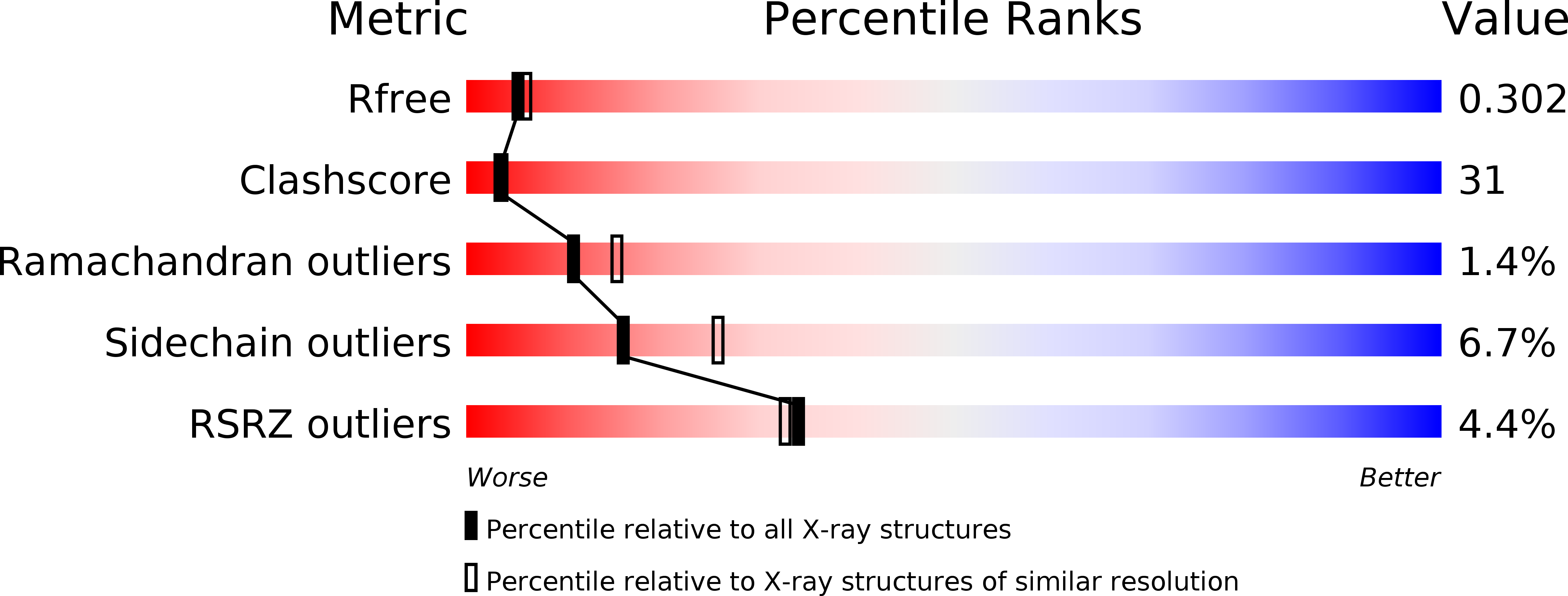
3PH0 - PubMed Abstract:
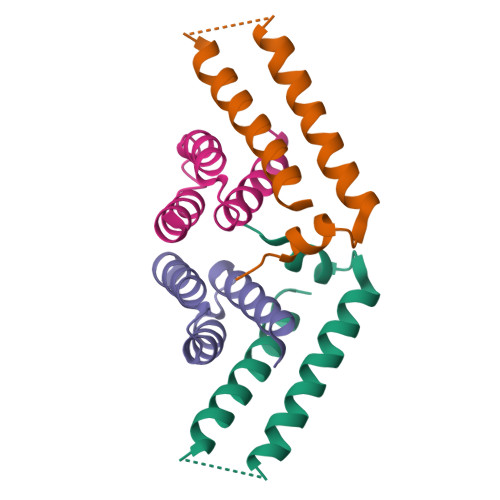
The putative needle complex subunit AscF forms a ternary complex with the chaperones AscE and AscG in the type III secretion system of Aeromonas hydrophila so as to avoid premature assembly. Previously, we demonstrated that the C-terminal region of AscG (residues 62-116) in the hetero-molecular chaperone, AscE-AscG, is disordered and susceptible to limited protease digestion. Here, we report the crystal structure of the ordered AscG(1-61) region in complex with AscE at 2.4 Å resolution. Helices α2 and α3 of AscE in the AscE-AscG(1-61) complex assumes a helix-turn-helix conformation in an anti-parallel fashion similar to that in apo AscE. However, in the presence of AscG, an additional N-terminal helix α1 in AscE (residues 4-12) is observed. PscG or YscG in the crystal structures of PscE-PscF-PscG or YscE-YscF-YscG, respectively, assumes a typical tetratricopeptide repeat (TPR) fold with three TPR repeats and one C-terminal capping helix. By comparison, AscG in AscE-AscG(1-61) comprises three anti-parallel helices that resembles the N-terminal TPR repeats in the corresponding region of PscG or YscG in PscE-PscF-PscG or YscE-YscF-YscG. Thermal denaturation of AscE-AscG and AscE-AscG(1-61) complexes demonstrates that the C-terminal disordered region does not contribute to the thermal stability of the overall complex. The N-terminal region of the AscG in the AscE-AscG complex is ordered and assumes a structure similar to those in the corresponding regions of PscE-PscG-PscF or YscE-YscF-YscG complexes. While the C-terminal region of AscG in the AscE-AscG complex is disordered and will assume its structure only in the presence of the substrate AscF. We hypothesize that AscE act as a chaperone of the chaperone to keep AscG in a stable but partially disordered state for interaction with AscF.
Organizational Affiliation:
Department of Biological Sciences, National University of Singapore, Singapore.