Crystal Structure of the Archaeal Asparagine Synthetase: Interrelation with Aspartyl-tRNA and Asparaginyl-tRNA Synthetases.
Blaise, M., Frechin, M., Olieric, V., Charron, C., Sauter, C., Lorber, B., Roy, H., Kern, D.(2011) J Mol Biology 412: 437-452
- PubMed: 21820443
- DOI: https://doi.org/10.1016/j.jmb.2011.07.050
- Primary Citation of Related Structures:
3P8T, 3P8V, 3P8Y, 3REU, 3REX, 3RL6 - PubMed Abstract:
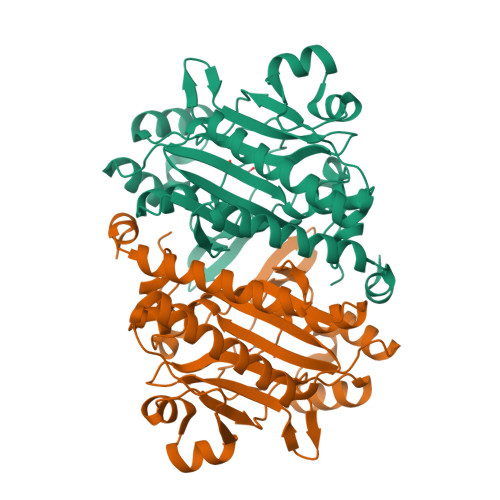
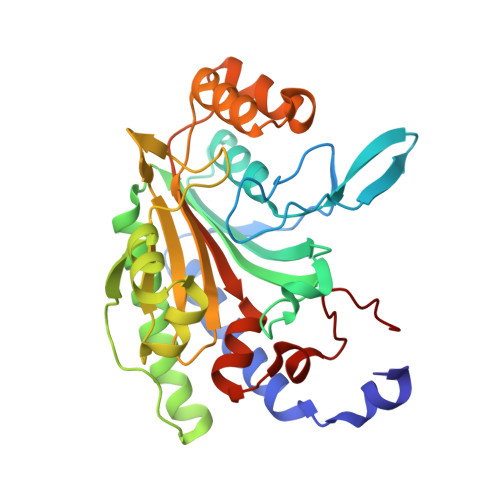
Asparagine synthetase A (AsnA) catalyzes asparagine synthesis using aspartate, ATP, and ammonia as substrates. Asparagine is formed in two steps: the β-carboxylate group of aspartate is first activated by ATP to form an aminoacyl-AMP before its amidation by a nucleophilic attack with an ammonium ion. Interestingly, this mechanism of amino acid activation resembles that used by aminoacyl-tRNA synthetases, which first activate the α-carboxylate group of the amino acid to form also an aminoacyl-AMP before they transfer the activated amino acid onto the cognate tRNA. In a previous investigation, we have shown that the open reading frame of Pyrococcus abyssi annotated as asparaginyl-tRNA synthetase (AsnRS) 2 is, in fact, an archaeal asparagine synthetase A (AS-AR) that evolved from an ancestral aspartyl-tRNA synthetase (AspRS). We present here the crystal structure of this AS-AR. The fold of this protein is similar to that of bacterial AsnA and resembles the catalytic cores of AspRS and AsnRS. The high-resolution structures of AS-AR associated with its substrates and end-products help to understand the reaction mechanism of asparagine formation and release. A comparison of the catalytic core of AS-AR with those of archaeal AspRS and AsnRS and with that of bacterial AsnA reveals a strong conservation. This study uncovers how the active site of the ancestral AspRS rearranged throughout evolution to transform an enzyme activating the α-carboxylate group into an enzyme that is able to activate the β-carboxylate group of aspartate, which can react with ammonia instead of tRNA.
Organizational Affiliation:
Architecture et Réactivité de l'ARN, Université de Strasbourg, CNRS, Institut de Biologie Moléculaire et Cellulaire, UPR 9002, 15 rue René Descartes, 67084 Strasbourg Cedex, France. mick@mb.au.dk