Trifluoromethylpyrimidine-based inhibitors of proline-rich tyrosine kinase 2 (PYK2): structure-activity relationships and strategies for the elimination of reactive metabolite formation.
Walker, D.P., Bi, F.C., Kalgutkar, A.S., Bauman, J.N., Zhao, S.X., Soglia, J.R., Aspnes, G.E., Kung, D.W., Klug-McLeod, J., Zawistoski, M.P., McGlynn, M.A., Oliver, R., Dunn, M., Li, J.C., Richter, D.T., Cooper, B.A., Kath, J.C., Hulford, C.A., Autry, C.L., Luzzio, M.J., Ung, E.J., Roberts, W.G., Bonnette, P.C., Buckbinder, L., Mistry, A., Griffor, M.C., Han, S., Guzman-Perez, A.(2008) Bioorg Med Chem Lett 18: 6071-6077
- PubMed: 18951788
- DOI: https://doi.org/10.1016/j.bmcl.2008.10.030
- Primary Citation of Related Structures:
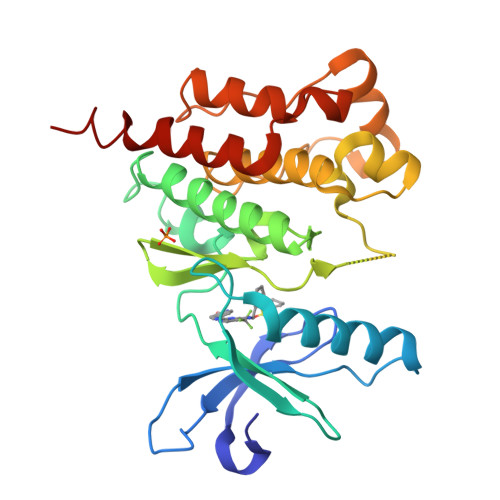
3ET7 - PubMed Abstract:
The synthesis and SAR for a series of diaminopyrimidines as PYK2 inhibitors are described. Using a combination of library and traditional medicinal chemistry techniques, a FAK-selective chemical series was transformed into compounds possessing good PYK2 potency and 10- to 20-fold selectivity against FAK. Subsequent studies found that the majority of the compounds were positive in a reactive metabolite assay, an indicator for potential toxicological liabilities. Based on the proposed mechanism for bioactivation, as well as a combination of structure-based drug design and traditional medicinal chemistry techniques, a follow-up series of PYK2 inhibitors was identified that maintained PYK2 potency, FAK selectivity and HLM stability, yet were negative in the RM assay.
Organizational Affiliation:
Pfizer Global Research and Development, Eastern Point Road, Groton, CT 06340, USA. daniel.p.walker@pfizer.com