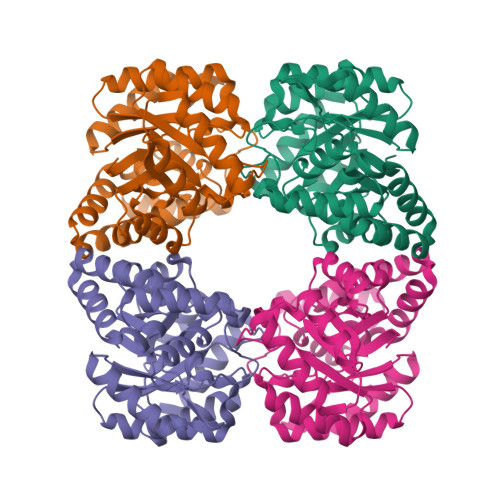
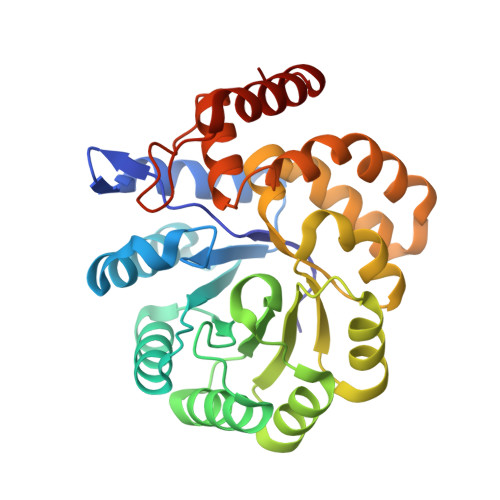
Structure of dihydrodipicolinate synthase from Methanocaldococcus jannaschii.
Padmanabhan, B., Strange, R.W., Antonyuk, S.V., Ellis, M.J., Hasnain, S.S., Iino, H., Agari, Y., Bessho, Y., Yokoyama, S.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 1222-1226
- PubMed: 20054116
- DOI: https://doi.org/10.1107/S174430910904651X
- Primary Citation of Related Structures:
2YXG - PubMed Abstract:
In bacteria and plants, dihydrodipicolinate synthase (DHDPS) plays a key role in the (S)-lysine biosynthesis pathway. DHDPS catalyzes the first step of the condensation of (S)-aspartate-beta-semialdehyde and pyruvate to form an unstable compound, (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinic acid. The activity of DHDPS is allosterically regulated by (S)-lysine, a feedback inhibitor. The crystal structure of DHDPS from Methanocaldococcus jannaschii (MjDHDPS) was solved by the molecular-replacement method and was refined to 2.2 A resolution. The structure revealed that MjDHDPS forms a functional homotetramer, as also observed in Escherichia coli DHDPS, Thermotoga maritima DHDPS and Bacillus anthracis DHDPS. The binding-site region of MjDHDPS is essentially similar to those found in other known DHDPS structures.
Organizational Affiliation:
Systems and Structural Biology Center, Yokohama Institute, RIKEN, 1-7-22 Suehiro, Tsurumi, Yokohama 230-0045, Japan. bpadmanabhan@hotmail.com