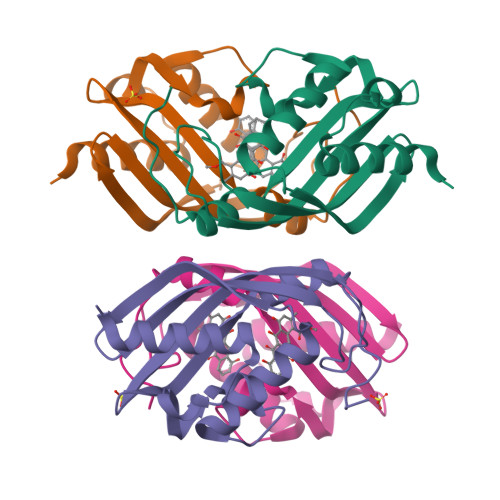
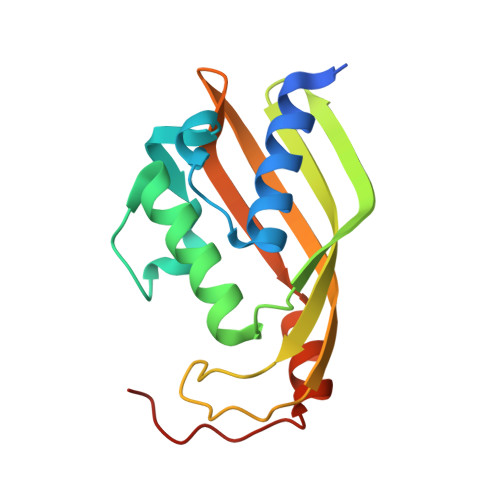
Crystal structure of the polyketide cyclase AknH with bound substrate and product analogue: implications for catalytic mechanism and product stereoselectivity.
Kallio, P., Sultana, A., Niemi, J., Schneider, G.(2006) J Mol Biology 357: 210-220
- PubMed: 16414075
- DOI: https://doi.org/10.1016/j.jmb.2005.12.064
- Primary Citation of Related Structures:
2F98, 2F99 - PubMed Abstract:
AknH is a small polyketide cyclase that catalyses the closure of the fourth carbon ring in aclacinomycin biosynthesis in Streptomyces galilaeus, converting aklanonic acid methyl ester to aklaviketone. The crystal structure analysis of this enzyme, in complex with substrate and product analogue, showed that it is closely related in fold and mechanism to the polyketide cyclase SnoaL that catalyses the corresponding reaction in the biosynthesis of nogalamycin. Similarity is also apparent at a functional level as AknH can convert nogalonic acid methyl ester, the natural substrate of SnoaL, to auraviketone in vitro and in constructs in vivo. Despite the conserved structural and mechanistic features between these enzymes, the reaction products of AknH and SnoaL are stereochemically distinct. Supplied with the same substrate, AknH yields a C9-R product, like most members of this family of polyketide cyclases, whereas the product of SnoaL has the opposite C9-S stereochemistry. A comparison of high-resolution crystal structures of the two enzymes combined with in vitro mutagenesis studies revealed two critical amino acid substitutions in the active sites, which contribute to product stereoselectivity in AknH. Replacement of residues Tyr15 and Asn51 of AknH, located in the vicinity of the main catalytic residue Asp121, by their SnoaL counter-parts phenylalanine and leucine, respectively, results in a complete loss of product stereoselectivity.
Organizational Affiliation:
Department of Biochemistry and Food Chemistry, University of Turku, FIN-20014 Turku, Finland.