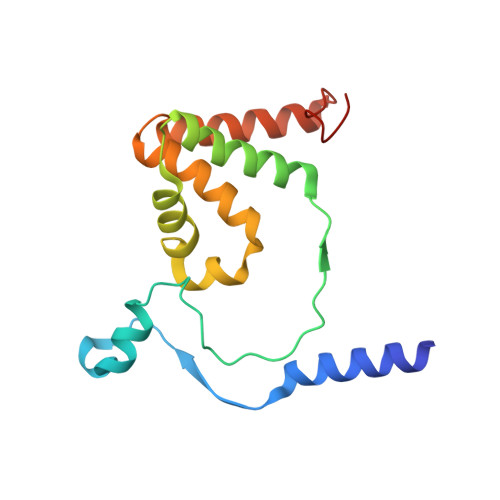
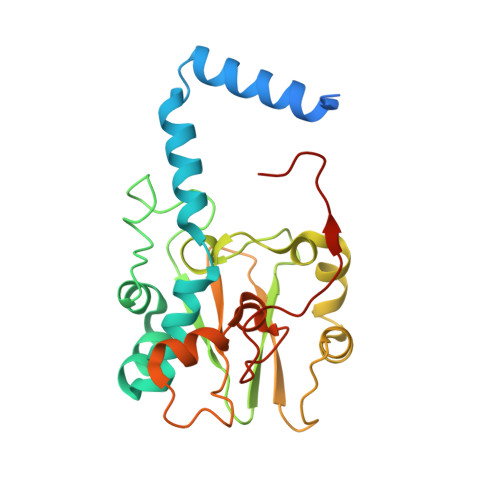
Structure of thiocyanate hydrolase: a new nitrile hydratase family protein with a novel five-coordinate cobalt(III) center.
Arakawa, T., Kawano, Y., Kataoka, S., Katayama, Y., Kamiya, N., Yohda, M., Odaka, M.(2007) J Mol Biology 366: 1497-1509
- PubMed: 17222425
- DOI: https://doi.org/10.1016/j.jmb.2006.12.011
- Primary Citation of Related Structures:
2DD4, 2DD5 - PubMed Abstract:
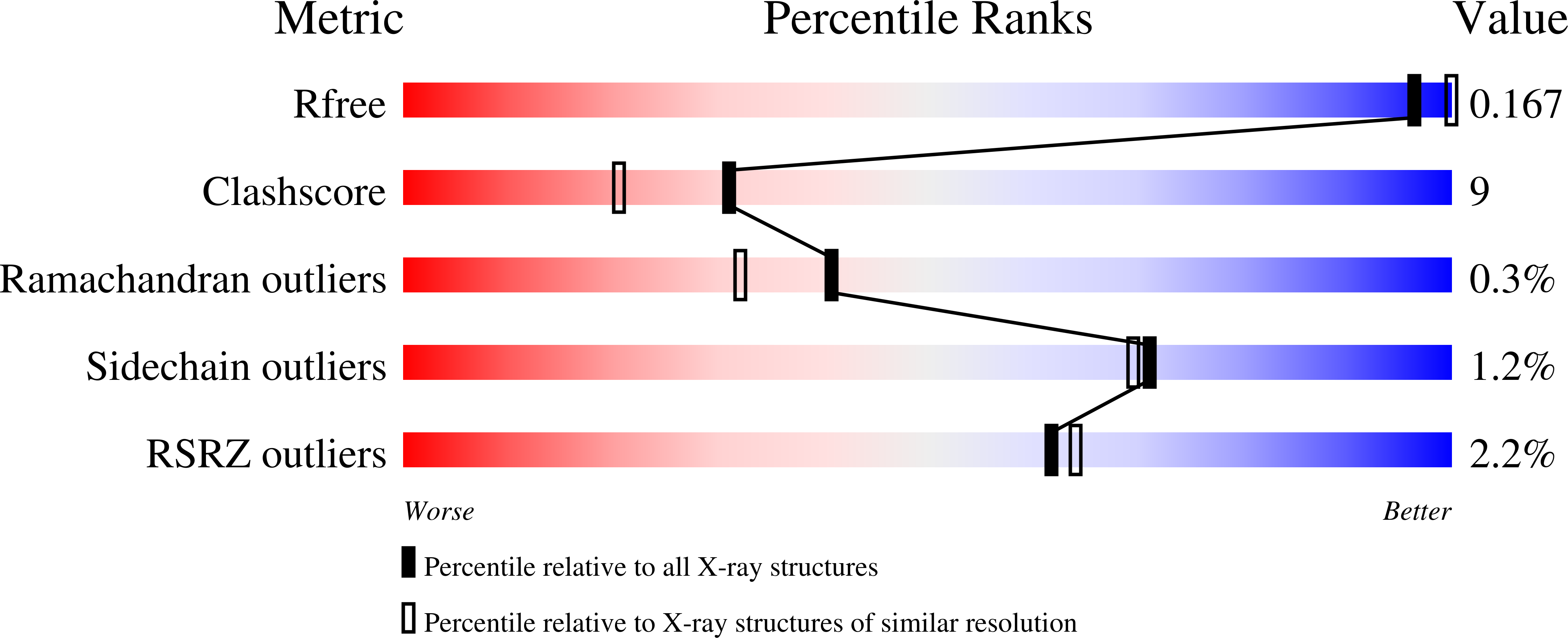
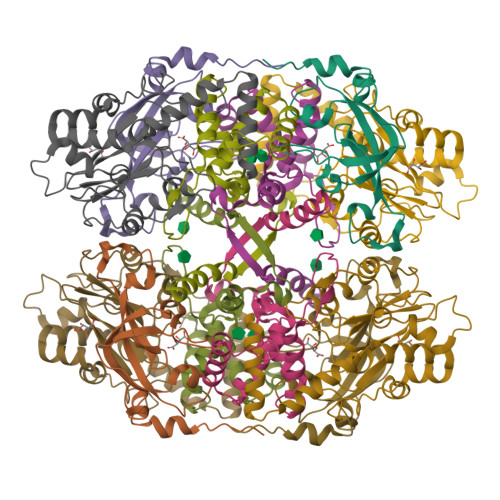
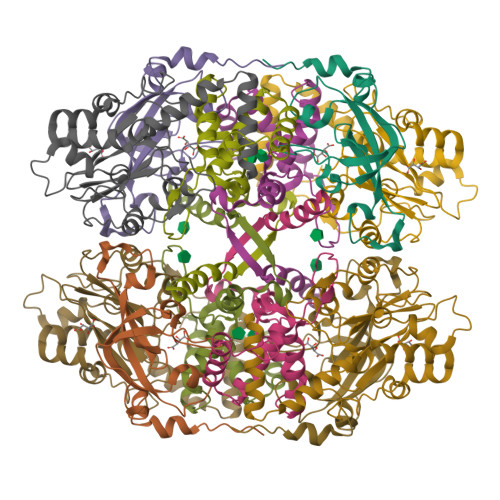
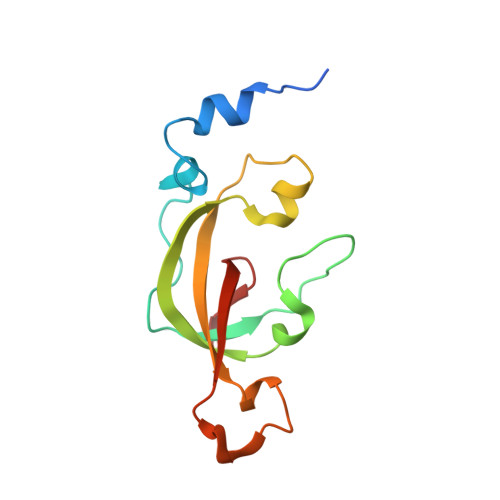
Thiocyanate hydrolase (SCNase) of Thiobacillus thioparus THI115 is a cobalt(III)-containing enzyme catalyzing the degradation of thiocyanate to carbonyl sulfide and ammonia. We determined the crystal structures of the apo- and native SCNases at a resolution of 2.0 A. SCNases in both forms had a conserved hetero-dodecameric structure, (alphabetagamma)(4). Four alphabetagamma hetero-trimers were structurally equivalent. One alphabetagamma hetero-trimer was composed of the core domain and the betaN domain, which was located at the center of the molecule and linked the hetero-trimers with novel quaternary interfaces. In both the apo- and native SCNases, the core domain was structurally conserved between those of iron and cobalt-types of nitrile hydratase (NHase). Native SCNase possessed the post-translationally modified cysteine ligands, gammaCys131-SO(2)H and gammaCys133-SOH like NHases. However, the low-spin cobalt(III) was found to be in the distorted square-pyramidal geometry, which had not been reported before in any protein. The size as well as the electrostatic properties of the substrate-binding pocket was totally different from NHases with respect to the charge distribution and the substrate accessibility, which rationally explains the differences in the substrate preference between SCNase and NHase.
Organizational Affiliation:
Department of Biotechnology and Life Science, Graduate School of Technology, Tokyo University of Agriculture and Technology, 2-24-16 Naka-cho, Koganei, Tokyo 184-8588, Japan.