Structure of the Male Determinant Factor for Brassica Self-incompatibility
Mishima, M., Takayama, S., Sasaki, K., Jee, J.G., Kojima, C., Isogai, A., Shirakawa, M.(2003) J Biological Chem 278: 36389-36395
- PubMed: 12835321
- DOI: https://doi.org/10.1074/jbc.M305305200
- Primary Citation of Related Structures:
1UGL - PubMed Abstract:
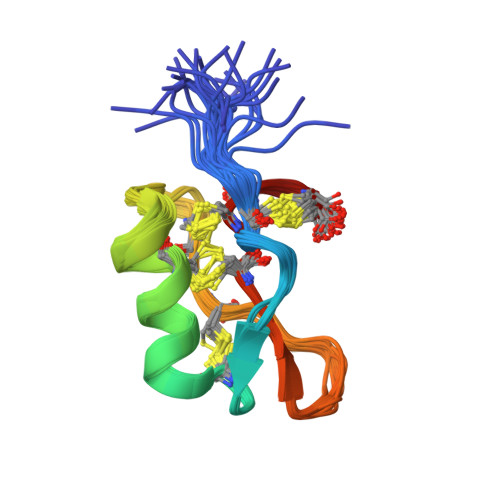
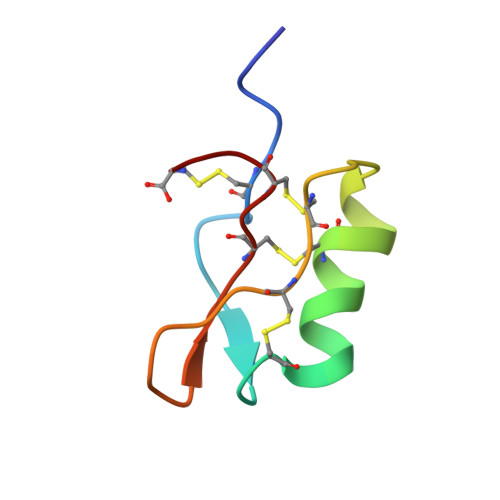
Many flowering plants possess a self-incompatibility system to prevent inbreeding. In Brassica rapa, self/non-self recognition in mating is established through S-haplotype-specific interactions between stigma receptors and S-locus protein 11 (SP11, also called S-locus cysteine-rich protein) that is encoded at the highly polymorphic S-locus. Here we describe the solution structure of the SP11 protein of the S8-haplotype (S8-SP11), which specifically binds to the stigma factor of the same haplotype. It folds into an alpha/beta sandwich structure that resembles those of plant defensins. Residues important for structural integrity are highly conserved among the allelic SP11s, suggesting the existence of a common folding pattern. Structure-based sequence alignment and homology modeling of allelic SP11 identified a hyper-variable (HV) region, which is thought to form a loop that bulges out from the body of the protein that is amenable to solvent exposure. We suggest that the HV region could serve as a specific binding site for the stigma receptor.
Organizational Affiliation:
Graduate School of Biological Sciences, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma 630-0101, Japan.