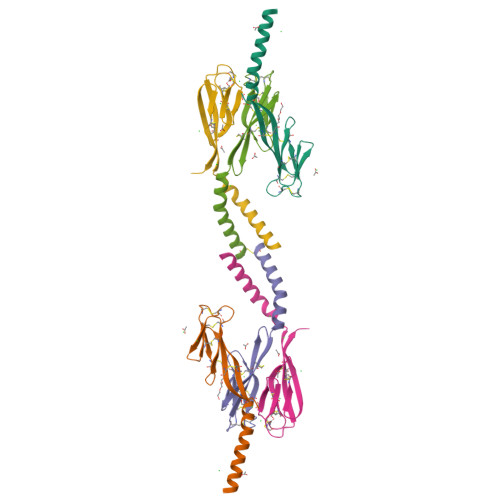
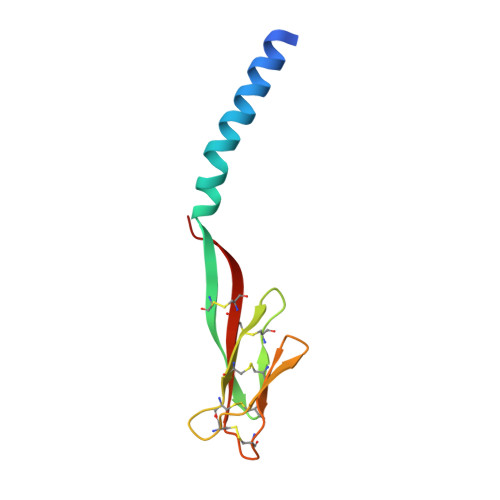
Disulfide-dependent multimeric assembly of resistin family hormones
Patel, S.D., Rajala, M.W., Rossetti, L., Scherer, P.E., Shapiro, L.(2004) Science 304: 1154-1158
- PubMed: 15155948
- DOI: https://doi.org/10.1126/science.1093466
- Primary Citation of Related Structures:
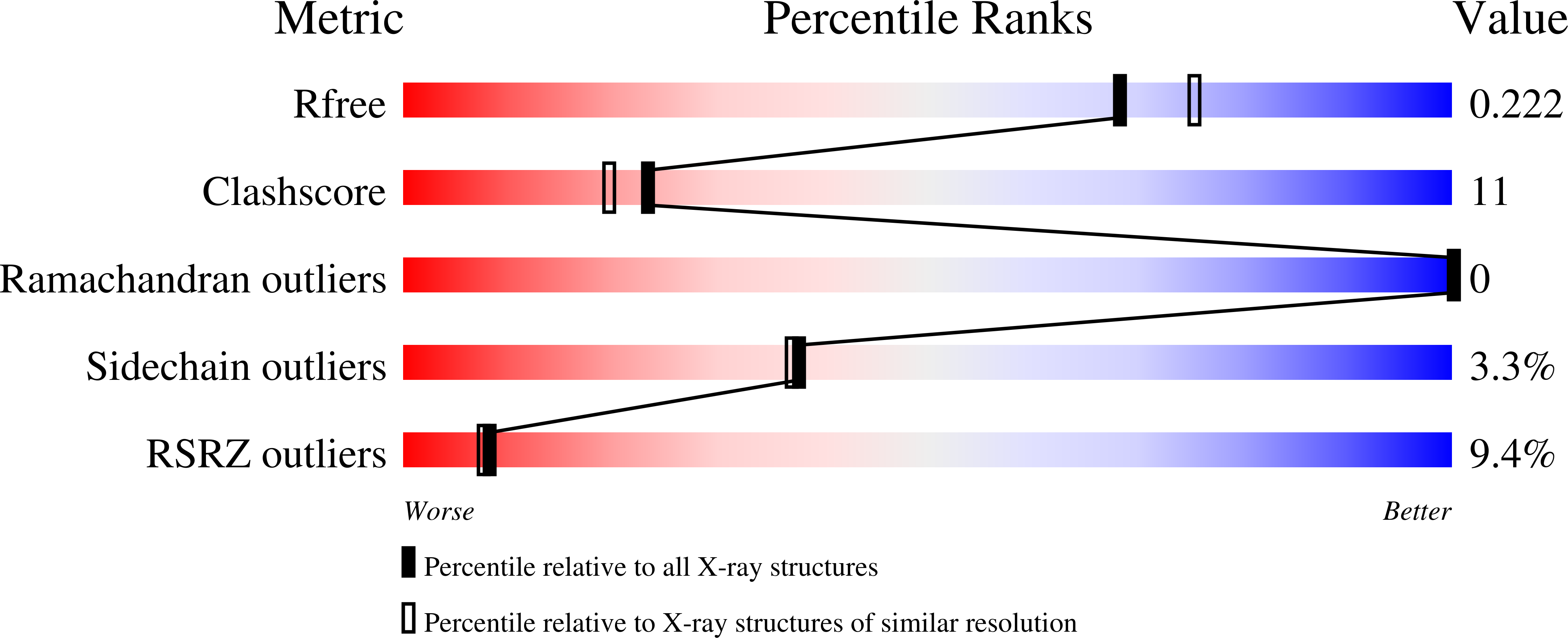
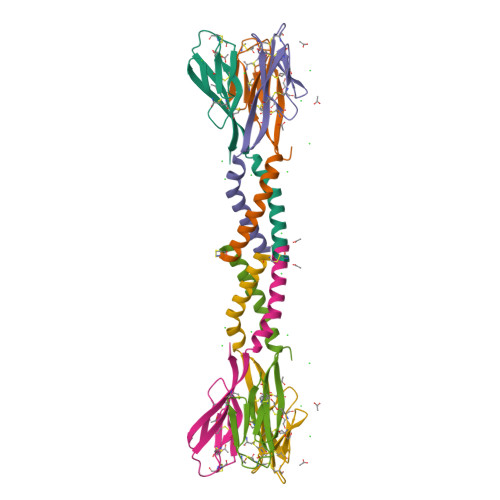
1RFX, 1RGX, 1RH7 - PubMed Abstract:
Resistin, founding member of the resistin-like molecule (RELM) hormone family, is secreted selectively from adipocytes and induces liver-specific antagonism of insulin action, thus providing a potential molecular link between obesity and diabetes. Crystal structures of resistin and RELMbeta reveal an unusual multimeric structure. Each protomer comprises a carboxy-terminal disulfide-rich beta-sandwich "head" domain and an amino-terminal alpha-helical "tail" segment. The alpha-helical segments associate to form three-stranded coiled coils, and surface-exposed interchain disulfide linkages mediate the formation of tail-to-tail hexamers. Analysis of serum samples shows that resistin circulates in two distinct assembly states, likely corresponding to hexamers and trimers. Infusion of a resistin mutant, lacking the intertrimer disulfide bonds, in pancreatic-insulin clamp studies reveals substantially more potent effects on hepatic insulin sensitivity than those observed with wild-type resistin. This result suggests that processing of the intertrimer disulfide bonds may reflect an obligatory step toward activation.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032, USA.