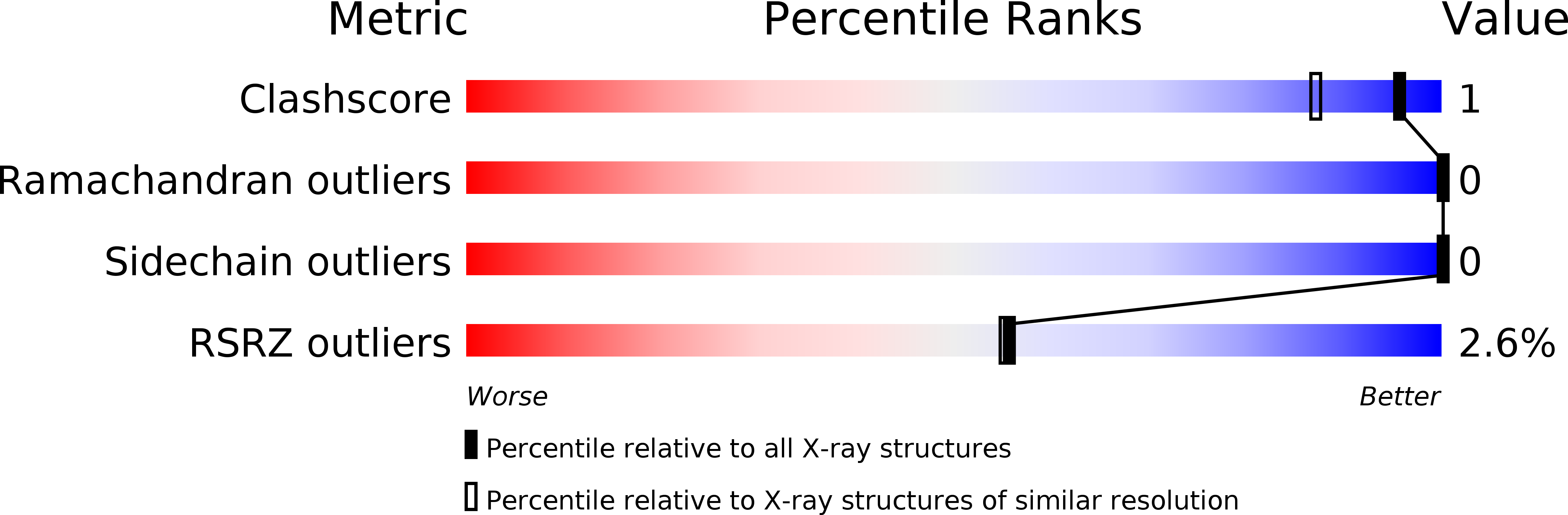
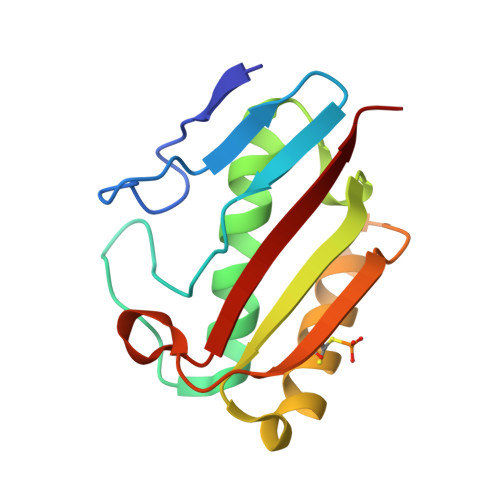
A test case for structure-based functional assignment: the 1.2 A crystal structure of the yjgF gene product from Escherichia coli
Volz, K.(1999) Protein Sci 8: 2428-2437
- PubMed: 10595546
- DOI: https://doi.org/10.1110/ps.8.11.2428
- Primary Citation of Related Structures:
1QU9 - PubMed Abstract:
The YER057c/YIL051c/YjgF protein family is a set of 24 full-length homologs, each approximately 130 residues in length, and each with no known function or relationship to proteins of known structure. To determine the function of this family, the structure of one member--the YjgF protein from Escherichia coli--was solved and refined at a resolution of 1.2 A. The YjgF molecule is a homotrimer with exact threefold symmetry. Its tertiary and quaternary structures are related to that of Bacillus subtilis chorismate mutase, although their active sites are completely different. The YjgF protein has an active site curiously similar to protein tyrosine phosphatases, including a covalently modified cysteine, but it is unlikely to be functionally related. The lessons learned from this attempt to deduce function from structure may be useful to future projects in structural genomics.
Organizational Affiliation:
Department of Microbiology and Immunology, University of Illinois at Chicago, 60612-7344, USA. [email protected]