Structural characterization and oligomerization of PB1-F2, a pro-apoptotic influenza A virus protein.
Bruns, K., Studtrucker, N., Sharma, A., Fossen, T., Mitzner, D., Eissmann, A., Tessmer, U., Roder, R., Henklein, P., Wray, V., Schubert, U.(2007) J Biological Chem 282: 353-363
- PubMed: 17052982
- DOI: https://doi.org/10.1074/jbc.M606494200
- Primary Citation of Related Structures:
2HN8 - PubMed Abstract:
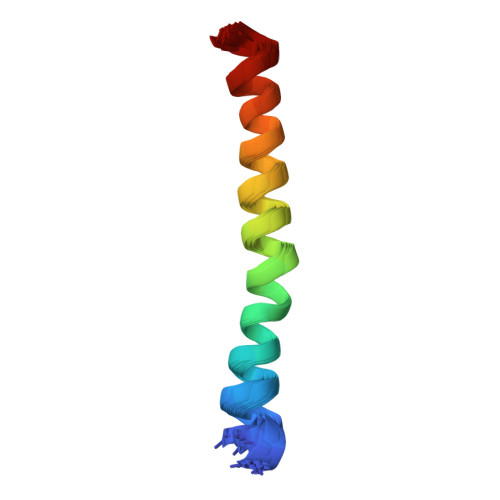
Recently, a novel 87-amino acid influenza A virus protein with proapoptotic properties, PB1-F2, has been reported that originates from an alternative reading frame in the PB1 polymerase gene and is encoded in most known human influenza A virus isolates. Here we characterize the molecular structure of a biologically active synthetic version of the protein (sPB1-F2). Western blot analysis, chemical cross-linking, and NMR spectroscopy afforded direct evidence of the inherent tendency of sPB1-F2 to undergo oligomerization mediated by two distinct domains located in the N and C termini, respectively. CD and (1)H NMR spectroscopic analyses indicate that the stability of structured regions in the molecule clearly depends upon the hydrophobicity of the solvent. In aqueous solutions, the behavior of sPB1-F2 is typical of a largely random coil peptide that, however, adopts alpha-helical structure upon the addition of membrane mimetics. (1)H NMR analysis of three overlapping peptides afforded, for the first time, direct experimental evidence of the presence of a C-terminal region with strong alpha-helical propensity comprising amino acid residues Ile(55)-Lys(85) connected via an essentially random coil structure to a much weaker helix-like region, located in the N terminus between residues Trp(9) and Lys(20). The C-terminal helix is not a true amphipathic helix and is more compact than previously predicted. It corresponds to a positively charged region previously shown to include the mitochondrial targeting sequence of PB1-F2. The consequences of the strong oligomerization and helical propensities of the molecule are discussed and used to formulate a hypothetical model of its interaction with the mitochondrial membrane.
Organizational Affiliation:
Institute of Clinical and Molecular Virology, University of Erlangen-Nürnberg, Erlangen D-91054, Germany.