Amyloid fibril structures and ferroptosis activation induced by ALS-causing SOD1 mutations.
Wang, L.Q., Ma, Y.Y., Zhang, M.Y., Yuan, H.Y., Li, X.N., Zhao, K., Chen, J., Li, D., Wang, Z.Z., Le, W.D., Liu, C., Liang, Y.(2024) Sci Adv
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2024) Sci Adv
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Superoxide dismutase [Cu-Zn] | 154 | Homo sapiens | Mutation(s): 1 Gene Names: SOD1 EC: 1.15.1.1 | 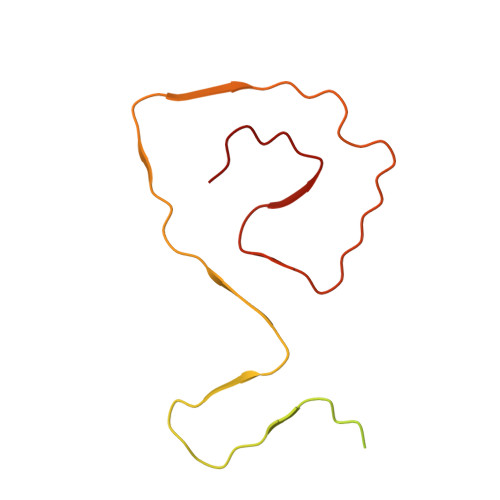 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00441 (Homo sapiens) Explore P00441 Go to UniProtKB: P00441 | |||||
PHAROS: P00441 GTEx: ENSG00000142168 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00441 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 32271326 |
| National Natural Science Foundation of China (NSFC) | China | 32071212 |
| National Natural Science Foundation of China (NSFC) | China | 31770833 |
| National Natural Science Foundation of China (NSFC) | China | 32201040 |