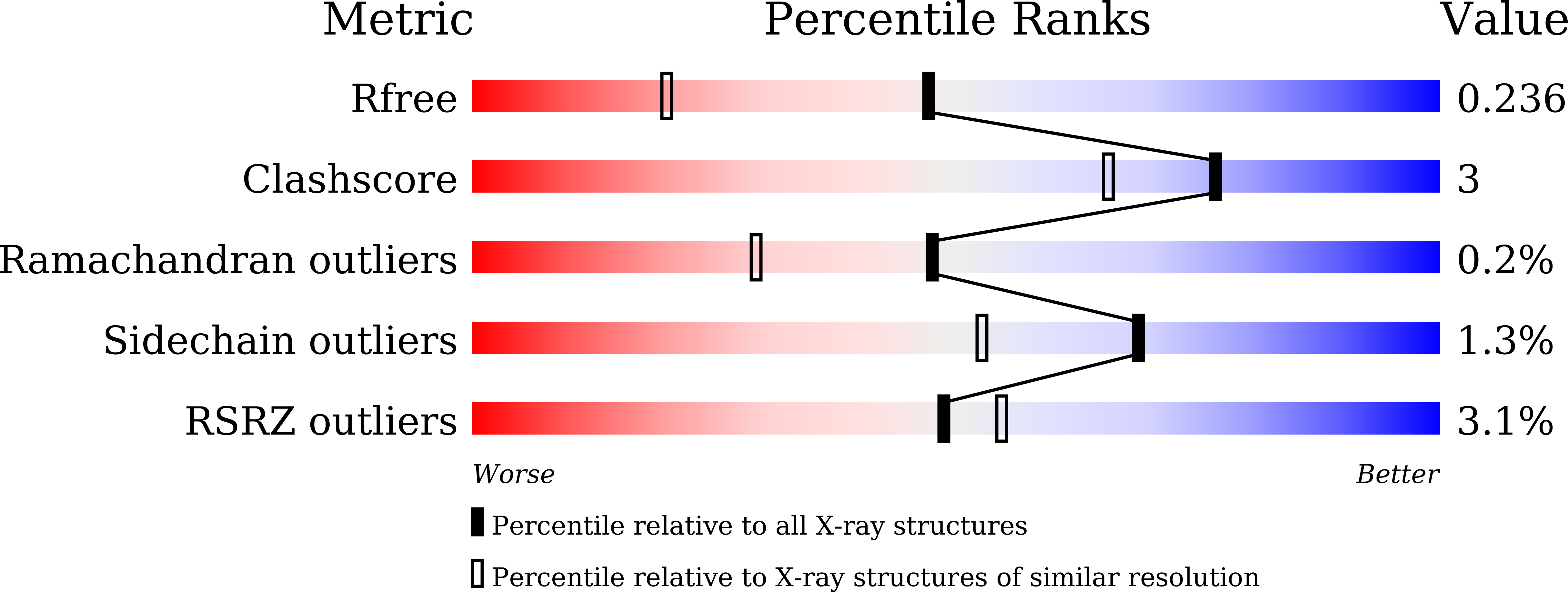
Crystal structures of main proteases of SARS-CoV-2 variants bound to a benzothiazole-based inhibitor.
Luo, J., Wang, W., Jiang, H., Li, W., Zeng, P., Wang, J., Zhou, X., Zou, X., Chen, S., Wang, Q., Zhang, J., Li, J.(2023) Acta Biochim Biophys Sin (Shanghai) 55: 1257-1264
- PubMed: 37357528
- DOI: https://doi.org/10.3724/abbs.2023053
- Primary Citation of Related Structures:
8HQF, 8HQG, 8HQH, 8HQI, 8HQJ - PubMed Abstract:
Main protease (M pro ) serves as an indispensable factor in the life cycle of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as well as its constantly emerging variants and is therefore considered an attractive target for antiviral drug development. Benzothiazole-based inhibitors targeting M pro have recently been investigated by several groups and proven to be promising leads for coronaviral drug development. In the present study, we determine the crystal structures of a benzothiazole-based inhibitor, YH-53, bound to M pro mutants from SARS-CoV-2 variants of concern (VOCs) or variants of interest (VOIs), including K90R (Beta, B.1.351), G15S (Lambda, C.37), Y54C (Delta, AY.4), M49I (Omicron, BA.5) and P132H (Omicron, B.1.1.529). The structures show that the benzothiazole group in YH-53 forms a C-S covalent bond with the sulfur atom of catalytic residue Cys145 in SARS-CoV-2 M pro mutants. Structural analysis reveals the key molecular determinants necessary for interaction and illustrates the binding mode of YH-53 to these mutant M pro s. In conclusion, structural insights from this study offer more information to develop benzothiazole-based drugs that are broader spectrum, more effective and safer.
Organizational Affiliation:
College of Pharmaceutical Sciences, Gannan Medical University, Ganzhou 341000, China.