Inhibition Analysis and High-Resolution Crystal Structure of Mus musculus Glutathione Transferase P1-1.
Kupreienko, O., Pouliou, F., Konstandinidis, K., Axarli, I., Douni, E., Papageorgiou, A.C., Labrou, N.E.(2023) Biomolecules 13
- PubMed: 37189361
- DOI: https://doi.org/10.3390/biom13040613
- Primary Citation of Related Structures:
8C5D - PubMed Abstract:
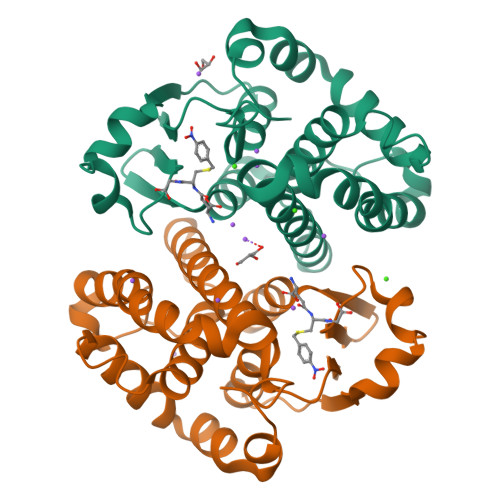
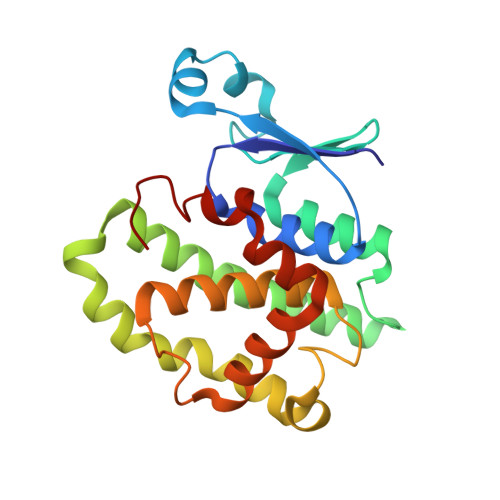
Multidrug resistance is a significant barrier that makes anticancer therapies less effective. Glutathione transferases (GSTs) are involved in multidrug resistance mechanisms and play a significant part in the metabolism of alkylating anticancer drugs. The purpose of this study was to screen and select a lead compound with high inhibitory potency against the isoenzyme GSTP1-1 from Mus musculus ( Mm GSTP1-1). The lead compound was selected following the screening of a library of currently approved and registered pesticides that belong to different chemical classes. The results showed that the fungicide iprodione [3-(3,5-dichlorophenyl)-2,4-dioxo-N-propan-2-ylimidazolidine-1-carboxamide] exhibited the highest inhibition potency (ΙC 50 = 11.3 ± 0.5 μΜ) towards Mm GSTP1-1. Kinetics analysis revealed that iprodione functions as a mixed-type inhibitor towards glutathione (GSH) and non-competitive inhibitor towards 1-chloro-2,4-dinitrobenzene (CDNB). X-ray crystallography was used to determine the crystal structure of Mm GSTP1-1 at 1.28 Å resolution as a complex with S-(p-nitrobenzyl)glutathione (Nb-GSH). The crystal structure was used to map the ligand-binding site of Mm GSTP1-1 and to provide structural data of the interaction of the enzyme with iprodione using molecular docking. The results of this study shed light on the inhibition mechanism of Mm GSTP1-1 and provide a new compound as a potential lead structure for future drug/inhibitor development.
Organizational Affiliation:
Laboratory of Enzyme Technology, Department of Biotechnology, School of Applied Biology and Biotechnology, Agricultural University of Athens, 75 Iera Odos Street, 11855 Athens, Greece.