Crystal structure of a novel alpha/beta hydrolase mutant from thermomonospora curvata in complex with pentane-1,5-diol
Han, X., Jian, G., Bornscheuer, U.T., Wei, R., Liu, W.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Triacylglycerol lipase | 260 | Thermomonospora curvata DSM 43183 | Mutation(s): 0 Gene Names: Tcur_1278 EC: 3.1.1.3 (PDB Primary Data), 3.1.1.101 (UniProt), 3.1.1.74 (UniProt) | 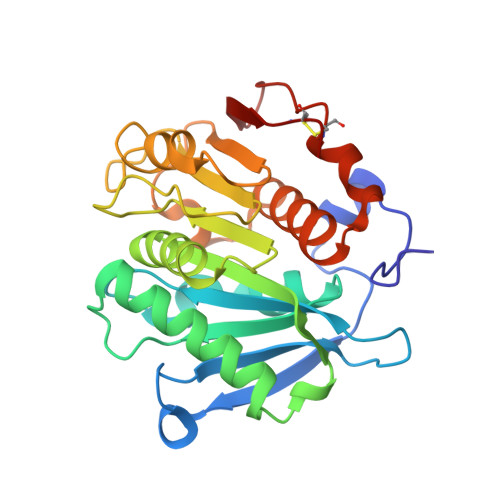 | |
UniProt | |||||
Find proteins for D1A9G5 (Thermomonospora curvata (strain ATCC 19995 / DSM 43183 / JCM 3096 / KCTC 9072 / NBRC 15933 / NCIMB 10081 / Henssen B9)) Explore D1A9G5 Go to UniProtKB: D1A9G5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | D1A9G5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 9JE (Subject of Investigation/LOI) Query on 9JE | B [auth A], C [auth A] | pentane-1,5-diol C5 H12 O2 ALQSHHUCVQOPAS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 45.66 | α = 90 |
| b = 71.6 | β = 90 |
| c = 143.22 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XSCALE | data scaling |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Chinese Scholarship Council | China | -- |
| Chinese Academy of Sciences | China | -- |