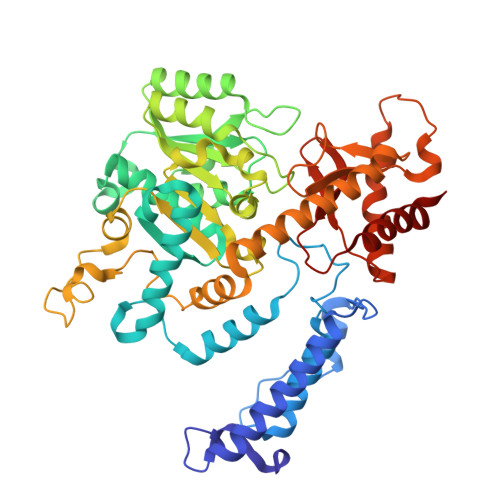
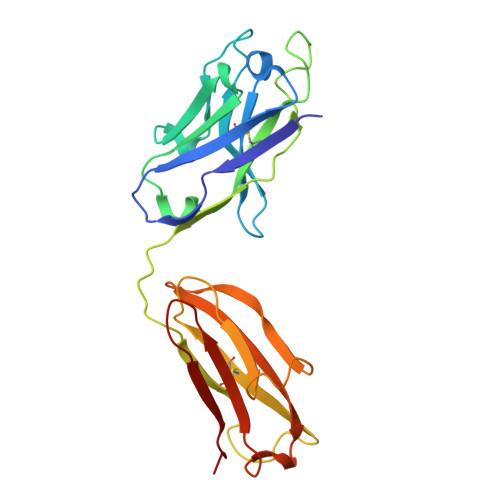
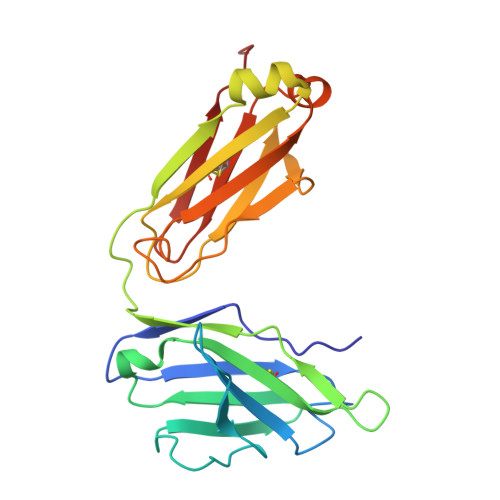
Structure and dynamics of the autoantigen GAD65 in complex with the human autoimmune polyendocrine syndrome type 2-associated autoantibody b96.11
Stander, S.H.D., Reboul, C.F., Le, S.N., Williams, D.E., Chandler, P.G., Costa, M.G.S., Hoke, D.E., Jimma, J.D.T., Fodor, J., Fenalti, G., Mannering, S.I., Porebski, B.T., Schofield, P., Christ, D., Buckle, M., McGowan, S., Elmlund, D., Rand, K.D., Buckle, A.M.(2024) bioRxiv