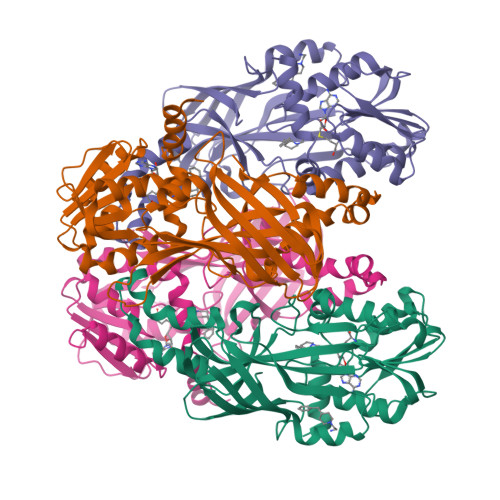
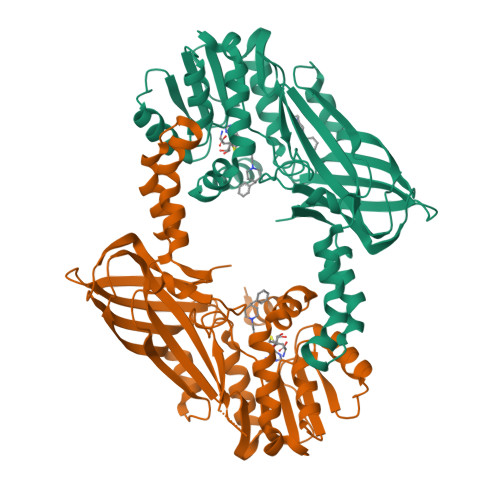
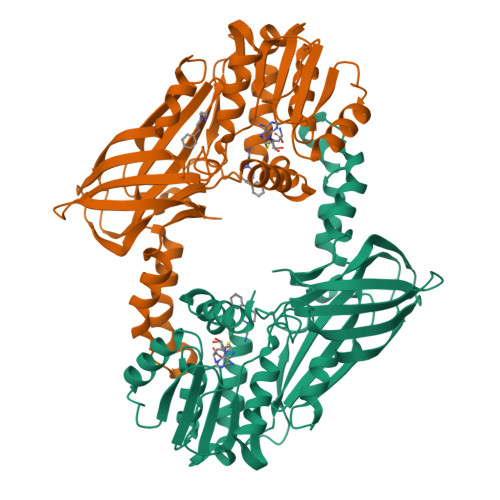
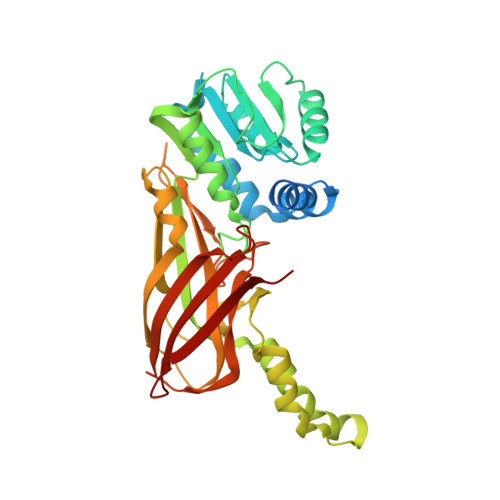
Discovery of a Potent Class I Protein Arginine Methyltransferase Fragment Inhibitor.
Ferreira de Freitas, R., Eram, M.S., Szewczyk, M.M., Steuber, H., Smil, D., Wu, H., Li, F., Senisterra, G., Dong, A., Brown, P.J., Hitchcock, M., Moosmayer, D., Stegmann, C.M., Egner, U., Arrowsmith, C., Barsyte-Lovejoy, D., Vedadi, M., Schapira, M.(2016) J Med Chem 59: 1176-1183
- PubMed: 26824386
- DOI: https://doi.org/10.1021/acs.jmedchem.5b01772
- Primary Citation of Related Structures:
5EGS - PubMed Abstract:
Protein methyltransferases (PMTs) are a promising target class in oncology and other disease areas. They are composed of SET domain methyltransferases and structurally unrelated Rossman-fold enzymes that include protein arginine methyltransferases (PRMTs). In the absence of a well-defined medicinal chemistry tool-kit focused on PMTs, most current inhibitors were identified by screening large and diverse libraries of leadlike molecules. So far, no successful fragment-based approach was reported against this target class. Here, by deconstructing potent PRMT inhibitors, we find that chemical moieties occupying the substrate arginine-binding site can act as efficient fragment inhibitors. Screening a fragment library against PRMT6 produced numerous hits, including a 300 nM inhibitor (ligand efficiency of 0.56) that decreased global histone 3 arginine 2 methylation in cells, and can serve as a warhead for the development of PRMT chemical probes.
Organizational Affiliation:
Structural Genomics Consortium, University of Toronto , Toronto, ON M5G 1L7, Canada.