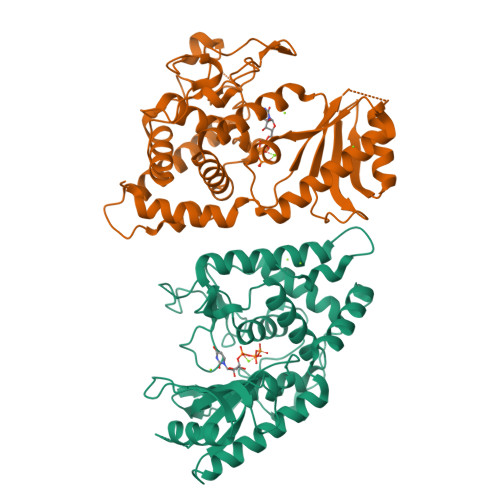
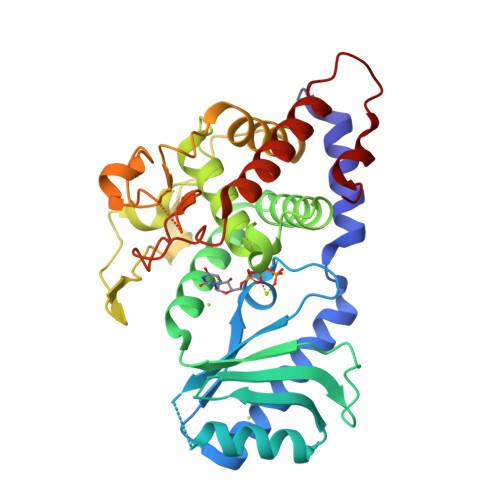
Functional implications from the cid1 poly(u) polymerase crystal structure.
Munoz-Tello, P., Gabus, C., Thore, S.(2012) Structure 20: 977-986
- PubMed: 22608966
- DOI: https://doi.org/10.1016/j.str.2012.04.006
- Primary Citation of Related Structures:
4EP7 - PubMed Abstract:
In eukaryotes, mRNA degradation begins with poly(A) tail removal, followed by decapping, and the mRNA body is degraded by exonucleases. In recent years, the major influence of 3'-end uridylation as a regulatory step within several RNA degradation pathways has generated significant attention toward the responsible enzymes, which are called poly(U) polymerases (PUPs). We determined the atomic structure of the Cid1 protein, the founding member of the PUP family, in its UTP-bound form, allowing unambiguous positioning of the UTP molecule. Our data also suggest that the RNA substrate accommodation and product translocation by the Cid1 protein rely on local and global movements of the enzyme. Supplemented by point mutations, the atomic model is used to propose a catalytic cycle. Our study underlines the Cid1 RNA binding properties, a feature with critical implications for miRNAs, histone mRNAs, and, more generally, cellular RNA degradation.
Organizational Affiliation:
Department of Molecular Biology, University of Geneva, 30 Quai Ernest Ansermet, Geneva 1211, Switzerland.