A Soluble Fucose-Specific Lectin from Aspergillus Fumigatus Conidia - Structure, Specificity and Possible Role in Fungal Pathogenicity.
Houser, J., Komarek, J., Kostlanova, N., Cioci, G., Varrot, A., Kerr, S.C., Lahmann, M., Balloy, V., Fahy, J.V., Chignard, M., Imberty, A., Wimmerova, M.(2013) PLoS One 8: 83077
- PubMed: 24340081
- DOI: https://doi.org/10.1371/journal.pone.0083077
- Primary Citation of Related Structures:
4AGI - PubMed Abstract:
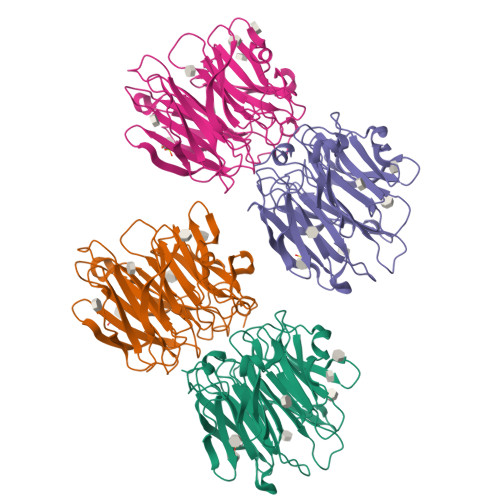
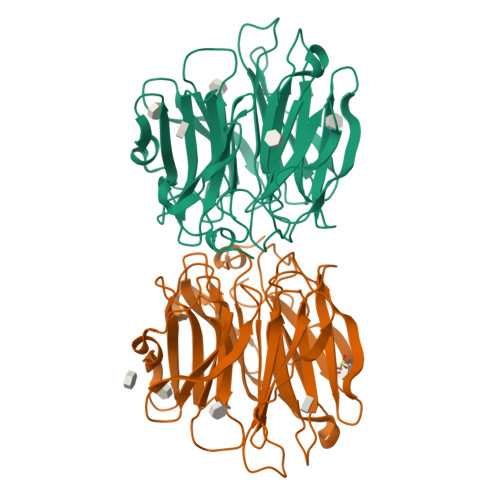
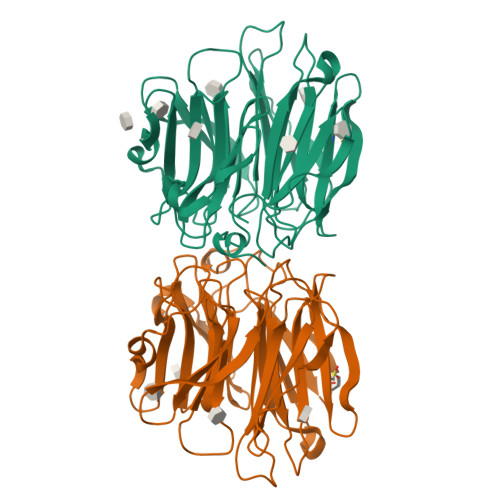
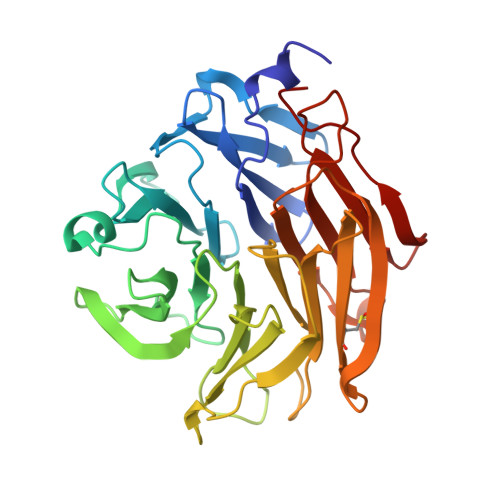
Aspergillus fumigatus is an important allergen and opportunistic pathogen. Similarly to many other pathogens, it is able to produce lectins that may be involved in the host-pathogen interaction. We focused on the lectin AFL, which was prepared in recombinant form and characterized. Its binding properties were studied using hemagglutination and glycan array analysis. We determined the specificity of the lectin towards l-fucose and fucosylated oligosaccharides, including α1-6 linked core-fucose, which is an important marker for cancerogenesis. Other biologically relevant saccharides such as sialic acid, d-mannose or d-galactose were not bound. Blood group epitopes of the ABH and Lewis systems were recognized, Le(Y) being the preferred ligand among others. To provide a correlation between the observed functional characteristics and structural basis, AFL was crystallized in a complex with methyl-α,L-selenofucoside and its structure was solved using the SAD method. Six binding sites, each with different compositions, were identified per monomer and significant differences from the homologous AAL lectin were found. Structure-derived peptides were utilized to prepare anti-AFL polyclonal antibodies, which suggested the presence of AFL on the Aspergillus' conidia, confirming its expression in vivo. Stimulation of human bronchial cells by AFL led to IL-8 production in a dose-dependent manner. AFL thus probably contributes to the inflammatory response observed upon the exposure of a patient to A. fumigatus. The combination of affinity to human epithelial epitopes, production by conidia and pro-inflammatory activity is remarkable and shows that AFL might be an important virulence factor involved in an early stage of A. fumigatus infection.
Organizational Affiliation:
Central European Institute for Technology, Masaryk University, Brno, Czech Republic ; National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Brno, Czech Republic.